Before we get started:
Biofilm formation is part of the reason S. mutans is so devastating to oral health (see Project Overview for more information). For adequate oral protection, biofilm formation is a problem we must solve, and this means we need a way to silence the appropriate proteins.
So how are proteins silenced? As we know, proteins are translated from single-stranded mRNAs. Just like eukaryotic cells, bacteria can selectively silence mRNA by transcribing sequences of sRNA, or bacterial small RNA.
These small RNA molecules are usually less than 50bp long, and function by binding to the specified, complementary mRNA strand to prevent ribosome attachment. We decided that targeted sRNA would give us a good shot at silencing HK11 and SGP in S. mutans.
Next up, we need to find some proteins to silence!
So how did we do it?
After careful literature search, we found two candidates:
Histidine kinase 11 (HK11) is a sensor kinase from a two-component signal transduction system. Literature indicates that a knock-out of HK11 resulted in dramatically decreased biofilm formation.
G protein in S. mutans (SGP) is involved in the regulation of intracellular GTP/GDP ratio, response to stress, and other diverse cellular functions. As with HK11, a knock-out of SGP resulted in dramatically decreased biofilm density.
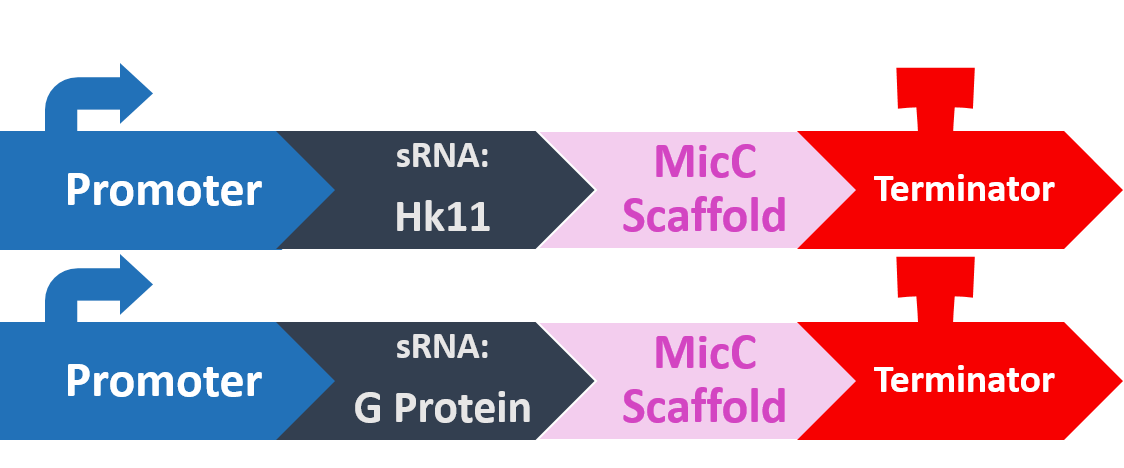
In order to silence these two proteins with sRNA, we synthesized a 24bp strand of non-coding DNA, and transcribed it into the corresponding RNA. This small RNA sequence is our artificial sRNA, and will bind to the TIR (translation initiation region) of our target mRNAs. This prevents the target mRNA from undergoing translation.
The final thing we need is something to help smooth out the whole process. The MicC scaffold is a specially constructed sequence which recruits the Hfq protein. The Hfq protein then helps our sRNA hybridize with its target mRNA, while stabilizing the sRNA-mRNA complex.
At last, our circuit is complete!
 "
"