Before we get started:
Although there are a plethora of oral bacteria, only a handful cause tooth decay, and chief among those is S. mutans (see [Project Overview] for more information). For our modified E. coli to deal with this threat, we need to find a way for them to get close to S. mutans colonies in the oral environment.
Competence Stimulating Peptide, or CSP, is an important quorum sensing (QS) pheromone specific to S. mutans, playing a huge role in their genetic response to population density. The system works since S. mutans continuously release CSP, which bind to the surface receptors of other S. mutans nearby. Check out [Control: Target] for more information on CSP.
The exclusivity of CSP and the ubiquity of its receptors in S. mutans makes it a great candidate for creating an anchoring module!
So how did we do it?
Because of size constraints, we did not want to use the actual CSP molecule itself. Instead, we found C16, a functionally active fragment of CSP which still binds to CSP receptors. Compared to CSP, C16 is smaller and more suitable for attachment on the E. coli surface.
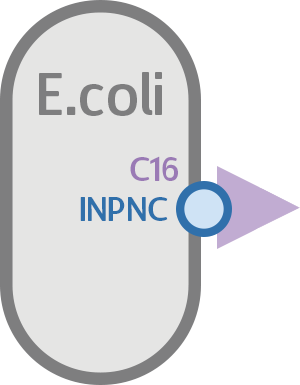
Now that the attachment candidate is found, what’s left is to express it on the surface of E. coli, so it can interact with S. mutans.
For that purpose, we found INPNC, a surface display protein in E. coli biobricked by the 2011 Edinburgh iGEM team. Using INPNC as basis, we created the INPNC-C16 fusion protein, which displays C16 on the surface of E. coli to achieve the results we want.
At last, our circuit is complete!

If everything works as planned, this circuit should express C16 on the surface of E. coli via INPNC, which will then bind to CSP receptors on S. mutans, anchoring the two cells together.

Putting it to the test!
Having a circuit is only the start! Now we must test how well our circuits function, specifically if INPNC can truly anchor the fusion protein on the cell membrane. The first test is a qualitative assay to determine if the fusion protein can truly function as intended, and the second is a quantitative test to further reinforce the conclusions of the first.
Is the INPNC-C16 fusion protein on the membrane?
To test whether or not our INPNC-C16 fusion protein can be successfully expressed on the surface of our E. coli, we constructed a testing circuit with RFP after C16:

We expect this circuit to produce an INPNC-C16-RFP fusion protein with RFP on the very outer side of the membrane, shown below.
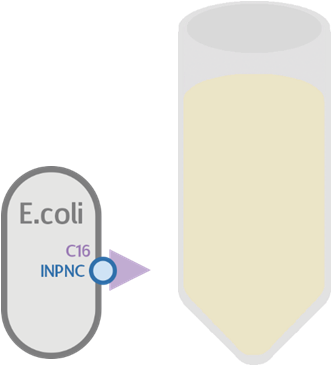
At the same time, we used our functional circuit design as a control:

We expect this circuit to produce everything identically, besides RFP, and that it will produce a colorless tube.
After constructing our testing circuit, we incubated the transformed bacteria together for 12 ~ 16 hours, before centrifugation. Theoretically, there are three possible results for our testing.
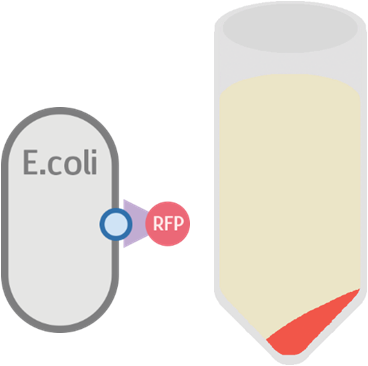
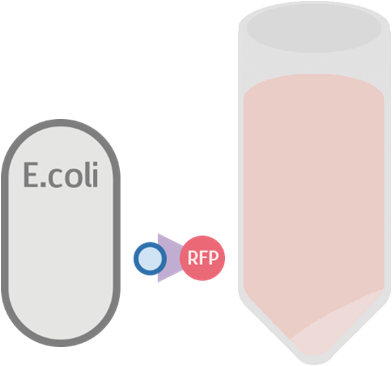
Possible Result 1: Fusion protein is on cell membrane
This is our anticipated result. If the fusion protein is on the membrane, then after centrifuge the pellet should display a very clear red color.
Possible Result 2: Fusion protein is inside cell membrane
If for some reason the fusion protein failed to display on the cell membrane, we will see a dull whitish-red color after centrifuge.
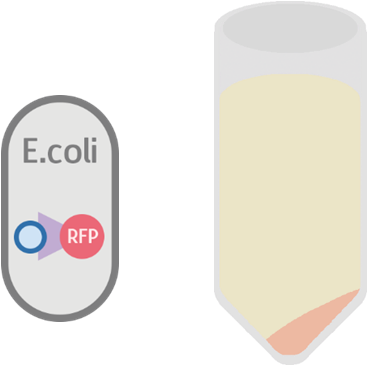
Possible Result 3: Fusion protein is outside cell membrane
If for some reason the fusion protein had been exported outside the cell, we will see a dull red color in the supernatant.
Here is the details of our method
- Use 5ml LB culture and 5μl chloramphenicol antibiotic to incubate bacteria transformed by function test circuit (K523013 + CSP16 + E1010 and B0015) and control circuit (K523013 + CSP16 + and B0015) 12~16 hour.
- Centrifuge 5cc bacteria culture at 13000 rps for 2 minute.
Our result turn out to be a clear red color! This indicates that our fusion protein has been successfully displayed on the surface of the cell membrane.
Testing the display function and efficiency of INPNC
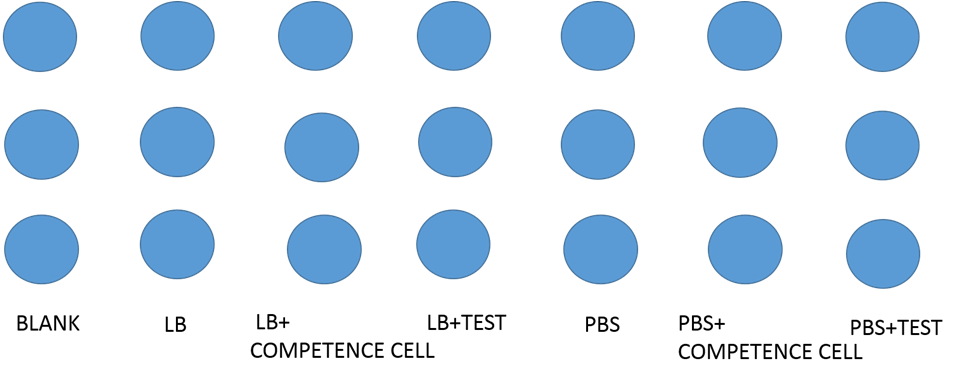
We designed the experiment below.
- Cultured our modified e-coli containing test circuit(promoter+INPNC+CSP16+RFP+terminator) in LB liquid culture for 12 hours.
- Used 96 well plate to culture above-mentioned e-coli.
- Used varioskan flash spectral scanning multimode reader to test fluorescence curve and OD curve every 2 hours.
- Also, compared culture in LB culture and PBS culture.
- Meanwhile, LB culture +competence cell and PBS culture+ competence cell as control.
Our results
First test
After centrifugation, the supernatant is clear; we could indicate that CSP16 is not secreted out of cell. Also, indicated from the red fluorescence of the pellet, we could say that INPNC protein display CSP16 and RFP on the surface.

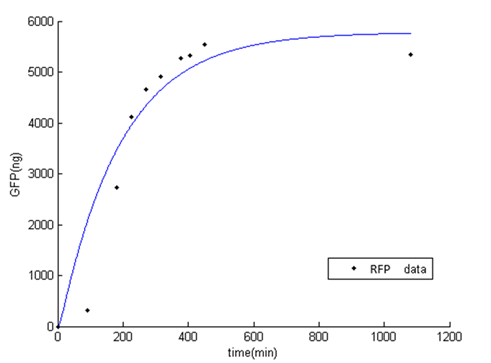
Second test
At the beginning, the population of E. coli is small as well as OD, so initial figure (Fluorescence /OD) is large. As time proceeds, Fluorescence /OD level increases dramatically, indicating that the INPNC protein is being greatly expressed. The OD level reaches a plateau at the end.
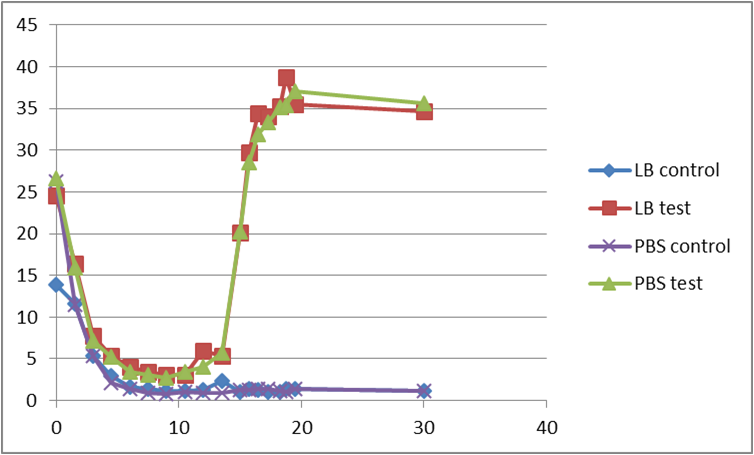
Fluorescence level /OD level indicates the display function of INPNC through growth time.
 "
"