Before we get started:
"Control" is so-called because it is used to control the number of S. mutans, instead of killing it completely off, which wouldn't help the matter as indicated in [Modeling: Competition]. When the number of S. mutans exceeds the threshold that causes cavities, the circuit will be activated, thus killing the excess S. mutans.
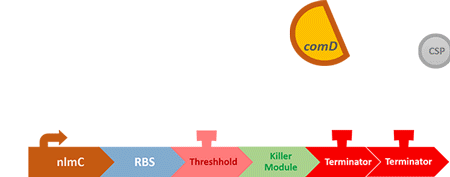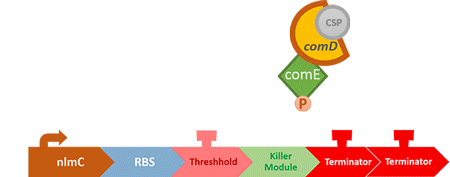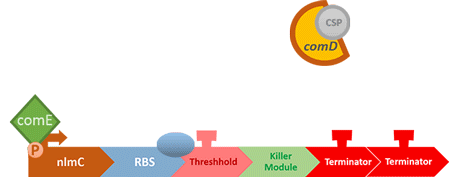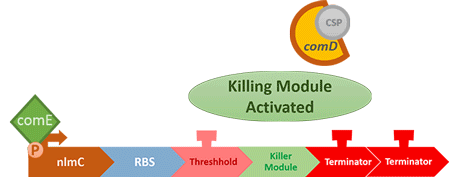
In order to find and confirm the threshold that causes cavities, we designed different kinds and numbers of terminators (each with different leakage rates) to create the threshold. Moreover, we combined both wet lab results and modelling to decide which design is more suitable.
Over the years, it has been found that the competence stimulating peptide, CSP, a quorum sensing chemical, is released in every competence S. mutans. Thus, we can detect the number of S. mutans by marking the amount of CSP.
In S. mutans the CSP bind to the membrane receptor, "comD", thereby phosphorylating the response regulator, "comE". The phosphorylated comE will activate numerous vital biological mechanisms in Streptococcus mutans such as biofilm formation and the release of mutacin, an antibiotic peptide. Compared with all the other mechanisms involved with CSP, it is found that the promoter of gene "nlmC"( non-lantibiotic mutacin C) in S. mutans has the best performance under the stimulation of CSP.
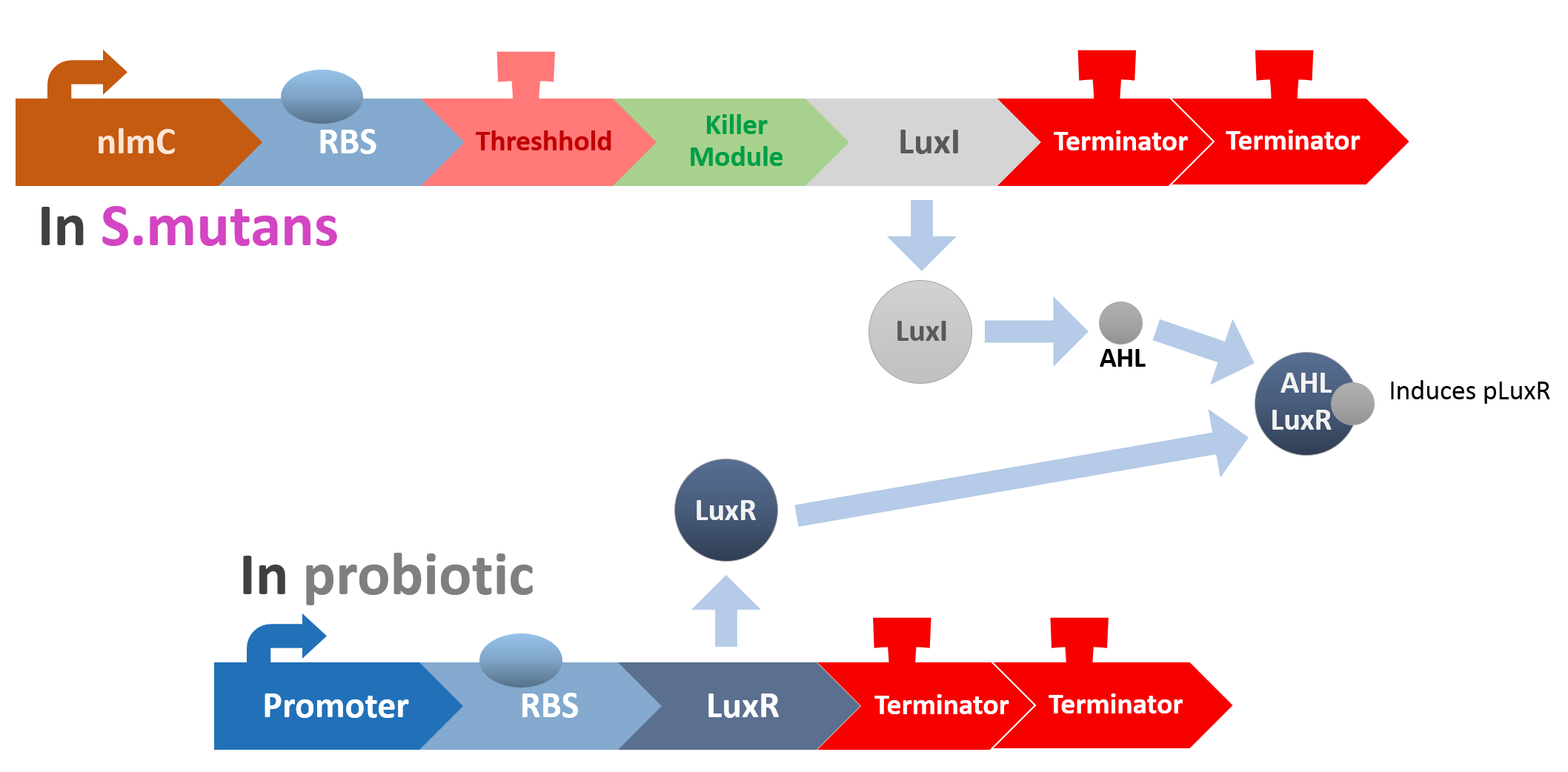
At this point, the two main organisms we adapted for our project are our probiotics and M102 bacteriophage. We used one of the most commonly seen quorum sensing chemicals, AHL, N-Acyl-homoserine-lactone, as our indicator between phage and E.coli. Due to the idea of phage threshold control of the number of S. mutans in prevention of the second-dominant strain in the oral cavity, we envision a possibility to trigger another killing system to reach synergistic effect on diminishing S. mutans. If the threshold terminators were passed, the downstream signaling pathway would be switched on and contact with the engineered probiotics.
When the amount of the S. mutans is so large that the phages couldn't afford the loading, CSP would pass the threshold value and turn on the luxR-luxI system. LuxI acts as a gene which would generate AHL-synthase that yielding the quorum-sensing signal AHL right after the threshold level of S. mutans is exceeded. LuxR is a protein which forms a complex with AHL, and then turns into an activated form which will afterwards bind with the promoter pLuxR and trigger it. The signal sequence and endolysin downstream would be secreted and eliminate the excess S. mutans.

Figure 1. Gram positive bacteria have only one layer of cell wall outside of cell membrane, composing of peptidoglycan, while the cell wall of gram negative bacteria has additional protection outside peptidoglycan cell wall.[1]
Endolysin is an enzyme expressed by phage-infected bacteria. The C-terminus bind to the host cell wall, while the N-terminus is enzymatic domain. Working with holin, which lyses cell membrane, endolysin could break the cell wall of bacteria. Usually serve as peptidase, endolysin tend to work on gram positive species, which have layers of peptidoglycan cell wall. Though originally intended to be utilized inside the cell wall, endolysin can also be applied outside cell according to previous research. Furthermore, it is even less harmful to gram negative bacteria this way because of the lipid layer of those bacteria composes cell wall.
So how did we do it?

In our circuit, we incorporate nlmC promoter and different combination of terminators to create the threshold.
When the amount of S. mutans is so large that the phages couldn't afford the loading, CSP would pass the threshold value and turn on the luxR-luxI system. LuxI in S. mutans will be triggered by nlmC and activate AHL which will combine with LuxR produced by probiotics continuously. When LuxR combine with AHL to form AHL-LuxR complex, it will induce pLuxR promoter and trigger the Killer Module.
Killer Module
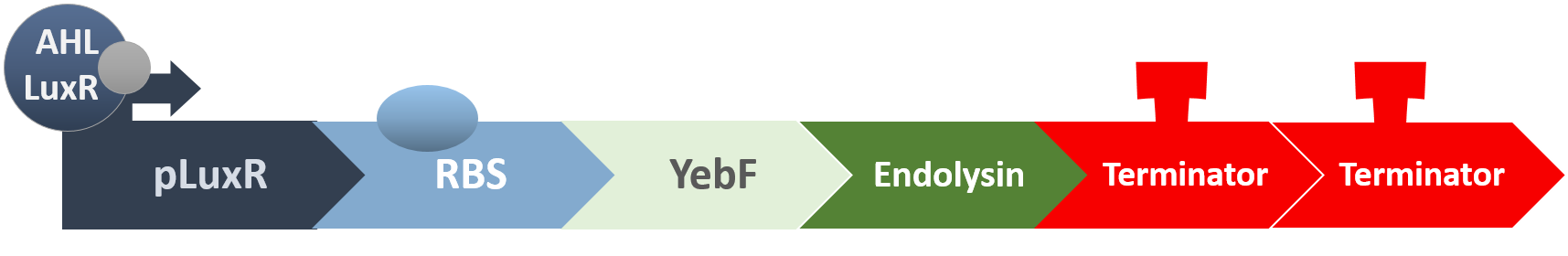
YebF is a protein that is naturally secreted by E.coli. It would transport proteins attached to it to the periplasm (space between cell membrane and cell wall), and later secrete it through porin on the cell wall. We chose YebF for its two advantages: one, it can be secreted in experimental strains which usually do not secrete proteins, along with other diverse passenger characters. Two, YebF can prevent endolysin from toxicating E. coli.
Putting it to the test!
Stage one: How well does nlmC function?
In order to test if nlmC functions as we expected it to, we constructed two circuits. The first is constructed with a constitutive promoter and GFP, and will serve as our control:
The other is constructed instead with nlmC and GFP, and will serve as the testing circuit for comparison between the efficacy of a constitutive promoter and the nlmC promoter:
To compare these circuits, we applied different amounts of synthetic CSP in different time lapses to our experimental group. If everything works as planned, the experimental group should display fluorescence in a wide array of intensity depending on CSP concentration, while the control group displays a uniform bright green.
Stage two: Which threshold combination should we use?
After confirming that the nlmC promoter works and responds to different levels of CSP, we need to design a test to determine which terminator combination we can best use as an effective threshold. We designed two circuits with different terminator thresholds, B0012 and double B0012, respectively, and GFP after them as a reporter.

The testing is similar to Stage 1, and we simply recorded the GFP production after induction with commercial CSP.
In the future, this protocol can be used to test many other combinations to find the "perfect" threshold for HOPE. After obtaining some experimental data, we employed extensive modeling to incorporate these numbers into other combinations of terminators, and we’ll be looking to validate these results after the Jamboree is over to further strengthen HOPE. Please see our [Model: Threshold] section for more information.
Stage three: Mechanisms of the Signal Receptor in our Probiotic
After all the fragments of the circuit is linked, we need a test to tell us that the fragments are well-connected, and that the mechanisms linking them has not lost its function. We created a circuit which is identical to our final circuit, except with RFP in place of the killing module:
We then transformed our circuit into E. coli, and added in commercial AHL. If the mechanisms remain robust after splicing, we should see a strong display of red from RFP.
Stage four: Mechanisms of the Signal Producer in S. mutans
One main concern with our mechanism is that AHL is a Gram-negative quorum sensing-signal… and yet we are implanting it into the Gram-positive S.mutans via M102. We need to test if the AHL production still works in S. mutans.
In this experiment, we first made a standard curve of the concentration of commercialized AHL versus absorption value. After that, we transformed the circuit containing LuxI into S. mutans. By extracting the supernatant of the culturing medium of the engineered S.mutans, and using a spectrometer to measure the concentration of AHL compared to the standard curve made previously, we can determine whether or not AHL is being expressed successfully in S. mutans.
Stage five: Does YebF work?
Before we check the functionality of the entire construct, we need to test the YebF protein for its ability to transport attached parts out of the membrane. We began by constructing two circuits:
These circuits are then transformed into E. coli, cultivated in liquid culture, and centrifuged. Afterwards, the supernatants are analyzed for their OD value. If YebF works as we expected, we should see the first circuit successfully secrete RFP into the supernatant :
While the control should only contain RFP which is not exported:
Stage six: Does Endolysin work?
To test the antimicrobial activity of endolysin, we also created two circuits:
The first circuit contains YebF and endolysin, which serves as the experimental circuit, and the second contains just YebF, and is the control. These are transformed into E. coli, and after culturing them overnight and extracting the supernatant, we spread the supernatant on plates of S. mutans to test for their antimicrobial activity.
Stage seven: Does AHL stimulate the killing program as anticipated?
In this stage, we put the final probiotic circuit into E. coli to see how well it performs.
We then incubated S. mutans together with the transformed E. coli and added commercial AHL to the mix. If everything works as expected, then the killing module should be successfully activated, and we should see a difference in S. mutans population.
Stage eight: Finally, let’s check Everything!
In this last stage we put all the constructed circuits intended for our probiotic and M102 into E. coli and S. mutans, respectively. Then we co-cultured these two transformed strains together to test our full circuit.
Our result
Reference
- http://www.expertsmind.com/topic/microbiology/bacterial-cell-wall-92313.aspx
 "
"