Team:SDU-Denmark/Tour40
From 2014.igem.org
Ulrikasimone (Talk | contribs) |
Ulrikasimone (Talk | contribs) |
||
| (22 intermediate revisions not shown) | |||
| Line 11: | Line 11: | ||
<p> | <p> | ||
| - | <span class="intro">The next few pages</span> | + | <span class="intro">The next few pages will</span> guide you through the results of the characterization of our submitted parts. On this page, you will find short descriptions of our results, leading you to the final result. We hope you will dig deeper into the results of our systems. After the need to prioritize subprojects, the aim of our project was to get <i>E. coli</i> to express a self-designed nutritional protein, controlled by an inducible promoter. Furthermore it will express limonene synthase for the synthesis of limonene, the main part of lemon flavor.<br><br> |
</p> | </p> | ||
| - | <h4>Synthesis of self-designed protein OneProt</h4> | + | <h4>Synthesis of self-designed protein OneProt</h4><br> |
| + | <div class="popupImg alignCenter" style="width:400px"> | ||
| + | <img src="https://static.igem.org/mediawiki/2014/4/4e/2014SDUWestern_blot_with_GroL.jpg" style="width:400px" /> | ||
| + | Figure 1: Western blot showing that the expression of OneProt can be detected. | ||
| + | </div><br> | ||
<p> | <p> | ||
| - | + | ||
| - | Growth curve<br><br> | + | <a class="popupImg alignRight" style="width:200px" target="_blank" href="https://static.igem.org/mediawiki/2014/b/bf/2014SDUGrowth_-_WT%2C_YYC912%2C_Oneprot%2C_Empty_vector.png" title="Figure 2: Growth curve illustrating the growth of <i>E. coli</i> K12 MG1655 expressing OneProt using a wild-type as control. "> |
| + | <img src="https://static.igem.org/mediawiki/2014/b/bf/2014SDUGrowth_-_WT%2C_YYC912%2C_Oneprot%2C_Empty_vector.png" style="width:200px" /> | ||
| + | Figure 2: Growth curve illustrating the growth of <i>E. coli</i> K12 MG1655 expressing OneProt using a wild-type as control. | ||
| + | </a> | ||
| + | |||
| + | <span class="intro">OneProt is a self-designed protein</span> containing the correct ratio of essential amino acids and the correct ratio between the essential and non-essential amino acids. Because the protein is self-designed, we wanted to detect if the protein was expressed in <i>E. coli</i> K12 MG1655 and if it stresses the cells and affects their growth. In order to test if the protein is expressed, we made a Western blot, taking advance of the FLAG-tag on OneProt. The Western blot shows that the protein is expressed; however, we cannot tell if the protein has been cut by proteases or not. <br><br> | ||
| + | |||
| + | <span class="intro">To test if the expression of the protein affects growth-rate</span>, we measured OD on <i>E. coli</i> expressing OneProt using a wild-type as control. The growth curve illustrates that the growth-rate of the cell expressing OneProt isn't affected much compared to that of the wild-type.<br><br> | ||
| + | |||
| + | <span class="intro">Even though we now know that OneProt is expressed</span> and that <i>E. coli</i> continues to grow, we want to make sure that the protein is not toxic upon digestion. In order for us to do so, we fed <i>Caenorhabditis elegans</i> (<i>C. elegans</i>) with <i>E. coli</i> K12 MG1655 containing an empty vector and a vector expressing OneProt on separate plates. To stress <i>C. elegans</i>, and thereby making it more sensitive, we used heat chock assay and stressed the organisms even more after 5 hours. The results are clear: after 7 hours of heat-shock assay, all <i>C.elegans</i> were alive, and thus we conclude that the OneProt has no toxic effects. <br><br> | ||
| + | <div class="popupImg alignCenter" style="width:300px" target="_blank" title="Figure 3: <i>C.elegans</i> fed with <i>E. coli</i> K12 MG1655 expressing OneProt. "> | ||
| + | <img src="https://static.igem.org/mediawiki/2014/b/bc/2014SDUresults10.jpg" style="width:300px" /> | ||
| + | Figure 3: <i>C.elegans</i> fed with <i>E. coli</i> K12 MG1655 expressing OneProt. | ||
| + | </div> | ||
| + | <br> | ||
</p> | </p> | ||
<h4>Controlling the Tet promoter</h4> | <h4>Controlling the Tet promoter</h4> | ||
<p> | <p> | ||
| - | <a class="popupImg alignRight" style="width:200px" target="_blank" href="https://static.igem.org/mediawiki/2014/4/4f/2014SDUresults5.png" title="Figure | + | <a class="popupImg alignRight" style="width:200px" target="_blank" href="https://static.igem.org/mediawiki/2014/4/4f/2014SDUresults5.png" title="Figure 5: Plate containing 200 ng/mL doxycycline plated with <i>E. coli</i> WT and <i>E. coli</i> expressing GFP controlled by a constitutive promoter, pTet-TeR(+LVA) and pTet-TetR(no LVA)."> |
<img src="https://static.igem.org/mediawiki/2014/1/19/2014SDUresults6.png" style="width:200px" /> | <img src="https://static.igem.org/mediawiki/2014/1/19/2014SDUresults6.png" style="width:200px" /> | ||
| - | Figure | + | Figure 5: Plate containing 200 ng/mL doxycycline plated with <i>E. coli</i> WT and <i>E. coli</i> expressing GFP controlled by a constitutive promoter, pTet-TeR(+LVA) and pTet-TetR(no LVA). |
</a> | </a> | ||
| - | <a class="popupImg alignRight" style="width:200px" target="_blank" href="https://static.igem.org/mediawiki/2014/b/b0/2014SDUresults3.png" title="Figure | + | <a class="popupImg alignRight" style="width:200px" target="_blank" href="https://static.igem.org/mediawiki/2014/b/b0/2014SDUresults3.png" title="Figure 4: Plate containing 0 ng/mL doxycycline plated with <i>E. coli</i> WT and <i>E. coli</i> expressing GFP controlled by a constitutive promoter, pTet-TeR(+LVA) and pTet-TetR(no LVA)."> |
<img src="https://static.igem.org/mediawiki/2014/e/e6/2014SDUresults4.png" style="width:200px" /> | <img src="https://static.igem.org/mediawiki/2014/e/e6/2014SDUresults4.png" style="width:200px" /> | ||
| - | Figure | + | Figure 4: Plate containing 0 ng/mL doxycycline plated with <i>E. coli</i> WT and <i>E. coli</i> expressing GFP controlled by a constitutive promoter, pTet-TeR(+LVA) and pTet-TetR(no LVA). |
</a> | </a> | ||
| - | <span class="intro">In order for us to</span> be able to control the amount of OneProt produced, we need a promoter that can be controlled. For this, we put OneProt under expressional control by pTet. Besides investigating the controllable expression, we also investigated the influence of the LVA tag on TetR. We proved that the inhibition of pTet by TetR is correlated with the concentration of inducer – increasing amounts of inducer means decreasing levels of inhibition of pTet. Comparing the expression by pTet controlled by TetR with and without LVA tag shows that there is a higher expression upon induction, using TetR with LVA than using TetR without LVA. We also show that the pTet is leaky. | + | <span class="intro">In order for us to</span> be able to control the amount of OneProt produced, we need a promoter that can be controlled. For this, we put OneProt under expressional control by pTet. Besides investigating the controllable expression, we also investigated the influence of the LVA tag on TetR. We proved that the inhibition of pTet by TetR is correlated with the concentration of inducer – increasing amounts of inducer means decreasing levels of inhibition of pTet. Comparing the expression by pTet controlled by TetR with and without LVA tag shows that there is a higher expression upon induction, using TetR with LVA than using TetR without LVA. We also show that the pTet is most likely leaky when on high-copy plasmids. |
</p> | </p> | ||
| - | <br><br><br> | + | <br><br><br><br> |
<div> | <div> | ||
| - | <a class="popupImg alignCenter" style="width:500px" target="_blank" href="https://static.igem.org/mediawiki/2014/1/12/2014SDUresults2.png" title="Figure | + | <a class="popupImg alignCenter" style="width:500px" target="_blank" href="https://static.igem.org/mediawiki/2014/1/12/2014SDUresults2.png" title="Figure 6: Dose response to doxycycline."> |
<img src="https://static.igem.org/mediawiki/2014/1/12/2014SDUresults2.png" style="width:500px" /> | <img src="https://static.igem.org/mediawiki/2014/1/12/2014SDUresults2.png" style="width:500px" /> | ||
| - | Figure | + | Figure 6: Dose response to doxycycline. |
</a><br> | </a><br> | ||
</div> | </div> | ||
| Line 43: | Line 61: | ||
<h4>Flavor improvement</h4> | <h4>Flavor improvement</h4> | ||
<p> | <p> | ||
| - | <span class="intro">The thought of eating | + | <span class="intro">The thought of eating <i>E. coli</i></span> does not sound that delicious – and that is why we want our OneProt to taste like lemon. Though the cloning proved unsuccessful. However, we have characterised an odor-free <i>E. coli</i> strain and compared it with a wild type K12 MG1655 <i>E. coli</i> strain. We have used Ion Mobility Spectroscopy to analyse the two strains, since we deemed it among the best methods to analyse odors. From the results it was shown that the odor-free strain does not produce indole, which is the compound responsible for the characteristic odor of <i>E. coli</i>. |
<br><br> | <br><br> | ||
</p> | </p> | ||
| Line 49: | Line 67: | ||
<h4>Added parts and devices</h4> | <h4>Added parts and devices</h4> | ||
<p> | <p> | ||
| - | <span class="intro">To the great iGEM Registry of Standard Biological Parts</span> we have added 3 basic parts, 2 regulatory devices and 4 regulable production devices. | + | <span class="intro">To the great iGEM Registry of Standard Biological Parts</span> we have added 3 basic parts, 2 regulatory devices, 1 constitutively active production device and 4 regulable production devices. |
<br><br><br> | <br><br><br> | ||
</p> | </p> | ||
Latest revision as of 03:46, 18 October 2014
Results
"I know that I am intelligent, because I know that I know nothing." – Socrates
The next few pages will guide you through the results of the characterization of our submitted parts. On this page, you will find short descriptions of our results, leading you to the final result. We hope you will dig deeper into the results of our systems. After the need to prioritize subprojects, the aim of our project was to get E. coli to express a self-designed nutritional protein, controlled by an inducible promoter. Furthermore it will express limonene synthase for the synthesis of limonene, the main part of lemon flavor.
Synthesis of self-designed protein OneProt
 Figure 1: Western blot showing that the expression of OneProt can be detected.
Figure 1: Western blot showing that the expression of OneProt can be detected.
 Figure 2: Growth curve illustrating the growth of E. coli K12 MG1655 expressing OneProt using a wild-type as control.
OneProt is a self-designed protein containing the correct ratio of essential amino acids and the correct ratio between the essential and non-essential amino acids. Because the protein is self-designed, we wanted to detect if the protein was expressed in E. coli K12 MG1655 and if it stresses the cells and affects their growth. In order to test if the protein is expressed, we made a Western blot, taking advance of the FLAG-tag on OneProt. The Western blot shows that the protein is expressed; however, we cannot tell if the protein has been cut by proteases or not.
Figure 2: Growth curve illustrating the growth of E. coli K12 MG1655 expressing OneProt using a wild-type as control.
OneProt is a self-designed protein containing the correct ratio of essential amino acids and the correct ratio between the essential and non-essential amino acids. Because the protein is self-designed, we wanted to detect if the protein was expressed in E. coli K12 MG1655 and if it stresses the cells and affects their growth. In order to test if the protein is expressed, we made a Western blot, taking advance of the FLAG-tag on OneProt. The Western blot shows that the protein is expressed; however, we cannot tell if the protein has been cut by proteases or not.
To test if the expression of the protein affects growth-rate, we measured OD on E. coli expressing OneProt using a wild-type as control. The growth curve illustrates that the growth-rate of the cell expressing OneProt isn't affected much compared to that of the wild-type.
Even though we now know that OneProt is expressed and that E. coli continues to grow, we want to make sure that the protein is not toxic upon digestion. In order for us to do so, we fed Caenorhabditis elegans (C. elegans) with E. coli K12 MG1655 containing an empty vector and a vector expressing OneProt on separate plates. To stress C. elegans, and thereby making it more sensitive, we used heat chock assay and stressed the organisms even more after 5 hours. The results are clear: after 7 hours of heat-shock assay, all C.elegans were alive, and thus we conclude that the OneProt has no toxic effects.
 Figure 3: C.elegans fed with E. coli K12 MG1655 expressing OneProt.
Figure 3: C.elegans fed with E. coli K12 MG1655 expressing OneProt.
Controlling the Tet promoter
 Figure 5: Plate containing 200 ng/mL doxycycline plated with E. coli WT and E. coli expressing GFP controlled by a constitutive promoter, pTet-TeR(+LVA) and pTet-TetR(no LVA).
Figure 5: Plate containing 200 ng/mL doxycycline plated with E. coli WT and E. coli expressing GFP controlled by a constitutive promoter, pTet-TeR(+LVA) and pTet-TetR(no LVA).
 Figure 4: Plate containing 0 ng/mL doxycycline plated with E. coli WT and E. coli expressing GFP controlled by a constitutive promoter, pTet-TeR(+LVA) and pTet-TetR(no LVA).
In order for us to be able to control the amount of OneProt produced, we need a promoter that can be controlled. For this, we put OneProt under expressional control by pTet. Besides investigating the controllable expression, we also investigated the influence of the LVA tag on TetR. We proved that the inhibition of pTet by TetR is correlated with the concentration of inducer – increasing amounts of inducer means decreasing levels of inhibition of pTet. Comparing the expression by pTet controlled by TetR with and without LVA tag shows that there is a higher expression upon induction, using TetR with LVA than using TetR without LVA. We also show that the pTet is most likely leaky when on high-copy plasmids.
Figure 4: Plate containing 0 ng/mL doxycycline plated with E. coli WT and E. coli expressing GFP controlled by a constitutive promoter, pTet-TeR(+LVA) and pTet-TetR(no LVA).
In order for us to be able to control the amount of OneProt produced, we need a promoter that can be controlled. For this, we put OneProt under expressional control by pTet. Besides investigating the controllable expression, we also investigated the influence of the LVA tag on TetR. We proved that the inhibition of pTet by TetR is correlated with the concentration of inducer – increasing amounts of inducer means decreasing levels of inhibition of pTet. Comparing the expression by pTet controlled by TetR with and without LVA tag shows that there is a higher expression upon induction, using TetR with LVA than using TetR without LVA. We also show that the pTet is most likely leaky when on high-copy plasmids.
Flavor improvement
The thought of eating E. coli does not sound that delicious – and that is why we want our OneProt to taste like lemon. Though the cloning proved unsuccessful. However, we have characterised an odor-free E. coli strain and compared it with a wild type K12 MG1655 E. coli strain. We have used Ion Mobility Spectroscopy to analyse the two strains, since we deemed it among the best methods to analyse odors. From the results it was shown that the odor-free strain does not produce indole, which is the compound responsible for the characteristic odor of E. coli.
Added parts and devices
To the great iGEM Registry of Standard Biological Parts we have added 3 basic parts, 2 regulatory devices, 1 constitutively active production device and 4 regulable production devices.
 "
"

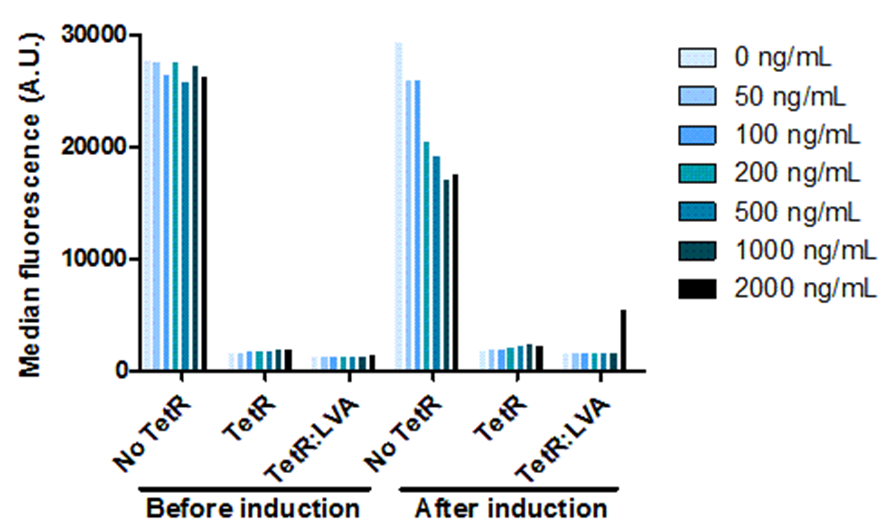 Figure 6: Dose response to doxycycline.
Figure 6: Dose response to doxycycline.