Team:Paris Saclay/Notebook/July/22
From 2014.igem.org
(→Preparation and transformation of competent cells MG1655 and MG1655Z1) |
m (→Reunion) |
||
| (2 intermediate revisions not shown) | |||
| Line 2: | Line 2: | ||
=Tuesday 22nd July= | =Tuesday 22nd July= | ||
==Lab Work== | ==Lab Work== | ||
| - | === | + | ===The chassis coli Odor free=== |
====Preparation and transformation of competent cells MG1655 and MG1655Z1 by electroporation==== | ====Preparation and transformation of competent cells MG1655 and MG1655Z1 by electroporation==== | ||
''by Arnaud'' | ''by Arnaud'' | ||
| Line 61: | Line 61: | ||
<span style="color:red">Note: we accidentally used a buffer without 10% ethanol. After removal of the buffer, we repeated the step with a correct version. This may have affected the electrophoresis which followed.</span> | <span style="color:red">Note: we accidentally used a buffer without 10% ethanol. After removal of the buffer, we repeated the step with a correct version. This may have affected the electrophoresis which followed.</span> | ||
| - | === | + | ===B - Lemon scent=== |
====Extraction of p cola plasmid DNA==== | ====Extraction of p cola plasmid DNA==== | ||
''by Sean'' | ''by Sean'' | ||
| Line 109: | Line 109: | ||
* Exhibition of our draft work | * Exhibition of our draft work | ||
| - | ''' | + | ==Photo of the Day== |
| - | * Instructors and advisors: Solenne. | + | [[File:Paris Saclay 22_july.jpg|600px|center]] |
| + | |||
| + | '''People there''': | ||
| + | * Instructors and advisors: Solenne and Sylvie. | ||
* Students: Arnaud, Fabio, Juliette, Mathieu, Marie, Pierre, Romain, Sean and Terry. | * Students: Arnaud, Fabio, Juliette, Mathieu, Marie, Pierre, Romain, Sean and Terry. | ||
| + | |||
{{Team:Paris_Saclay/notebook_footer}} | {{Team:Paris_Saclay/notebook_footer}} | ||
Latest revision as of 15:25, 14 October 2014
Contents |
Tuesday 22nd July
Lab Work
The chassis coli Odor free
Preparation and transformation of competent cells MG1655 and MG1655Z1 by electroporation
by Arnaud
Dilute 300µl of bacterial culture in 30ml LB medium at 30°C.
Shake vigorously at 30°C until the OD600nm reaches 0,6.
When the OD600 reached 0.6, proceed to the electroporation.
2 - Samples for stock
by Fabio
We collected 1ml of each sample, added 240µl of glycerol (87%) and stored at -80°C.
- BBa_K1033902
- BBa_K1033905
- BBa_K1033910
- BBa_K1033913
- BBa_K1033922
- BBa_K1033925
- BBa_K1033927
- BBa_K1033929
- BBa_K103921
- BBa_J45017
- BBa_K731201
- BBa_K762100
from Liquide Bacterial Cultures transformed the 21st July
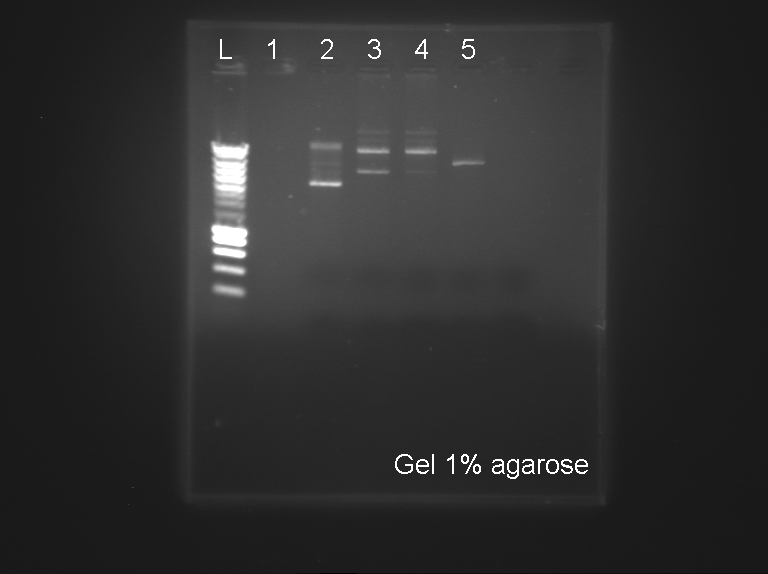
3 - Electrophoresis
by Arnaud (Process A), Fabio (gel), Marie and Romain (Process A)
We used 2µL of DNA of the following Biobricks in a 1% Agarose Gel.
Process A
- Empty
- p cola Ges
- DY330 pJBEI I
- DY330 pJBEI II
- BT340
Results:
- A:
4 - Bacterial Culture
by Fabio and Marie
One liquid culture with 5ml of LB (37°C - 150 rpm), IL1403 from the concentrated colony.
5 - Plasmid DNA extraction
by Sean and Terry
Plasmids used: cf part 1
Note: we accidentally used a buffer without 10% ethanol. After removal of the buffer, we repeated the step with a correct version. This may have affected the electrophoresis which followed.
B - Lemon scent
Extraction of p cola plasmid DNA
by Sean
p cola is a low-copy plasmid, and thus requires a slightly different protocol than other plasmids.
PCR Targeting
by Romain
Strains used: DY330 (clone I and II). Plasmid used: pJBEI-6409 (code for enzymes to produce limonene from glucose in E. coli)
Protocol: (For each clone)
Step 1 - Dilution of 300µl of bacterial culture in 30ml of LB + 30µl Cm at 30°C.
Step 2 - With the pre-culture:
- make glycerol stock: 1ml bacterial culture with 240µl glycerol 87%.
- Make extraction of 5ml of plasmid Protocol
Step 3 - When the culture OD650 = 0,6 (Step 1):
- put in ice during 10min.
- centriguge 4°C, 5min, 4000rpm.
- Discard the supernatant, and resuspend the pellet in 30ml glycerol 10% COLD.
- centriguge 4°C, 5min, 4000rpm.
- Discard the supernatant, and resuspend the pellet in 15ml glycerol 10% COLD.
- centriguge 4°C, 5min, 4000rpm.
- Discard the supernatant, and resuspend the pellet in 200µl glycerol 10% COLD.
Step 4 - Transformation:
- control 50µl (without DNA)
- 50µl transformed bacteria + 2,5µl plasmid + 7,5µl pOSV 230 PCR (purifié)
Step 5 - Electroporation control
Step 6 - Spread on ApraR and CmR dishes
- Control dishes : 100µl ND
- 1ml of bacterial culture (250µl on each dish)
Results: 24th July
Reunion
Conference with the team to discuss about the project. The handled topics were:
- Decisions about our new Visual Identity
- Order of the t-shirts and sweaters for the team
- Discussion about the main message of the project
- Presentation's planning of the project
- Exhibition of our draft work
Photo of the Day
People there:
- Instructors and advisors: Solenne and Sylvie.
- Students: Arnaud, Fabio, Juliette, Mathieu, Marie, Pierre, Romain, Sean and Terry.
 "
"