Team:Paris Saclay/Notebook/August/26
From 2014.igem.org
Tuesday 26th August
Lab work
B - Construction of the fusion protein
plasmid extraction : pGEMTeasy + chromoprotein
by Laetitia
We used the bacteria containing pGEMTeasy + chromoprotein launched by Laetitia monday the 25th for the plasmid extraction, 6 independent cultures which come from the 6 stocks made yesterday.
To extract the plasmid, we used the plasmid DNA purification kit (Macherey-Nagel). Protocol for low copy plasmid.
Digestion of pGEMTeasy+chromoproteinby XbaI and PstI
by Laetitia
The goal is to check the presence of the insert inside the plasmid. We digest the 6 samples of purified plasmid.
| component | volume |
|---|---|
| Plasmid | 10 μl |
| Fast Digest buffer 10X | 1,5 μl |
| XbaI | 0.5μl |
| PstI | 0.5μl |
| H20 | 2,5 μl |
3-4h at 37°C
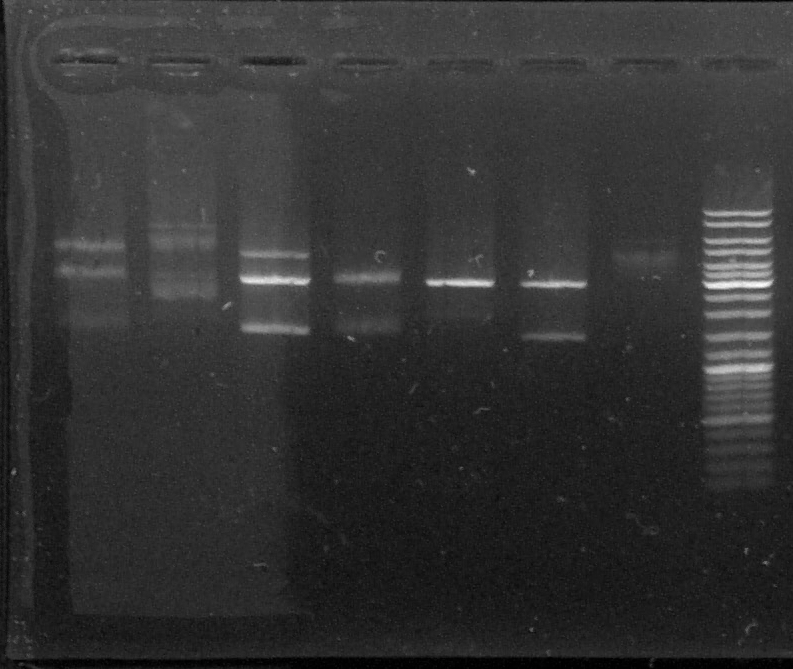
Electrophoresis of the digestion product of pGEMTeasy+chromoprotein
by Laetitia
T : control negatif without enzyme
| component | volume |
|---|---|
| blue | 2 μl |
| Plasmid | 2 μl |
| H20 | 8μl |
D : sample digested by XbaI and PstI
D1 - D2 - D3 - D4 - D5 - D6 - T - L
We can see two or three bands for the digested samples : the digestion has worked.
The band at 1500kb is the chromoprotein gene, the band at 3000kb is pGEMTeasy alone. The band at 4500kb is the plasmid containing the chromoprotein gene, non digested.
We decided to use the plasmid samples 3, 4 and 6
Digestion of pGEMTeasy+chromoprotein 3,4 and 6 by EcoRI and PstI
by Laetitia
We digest the remaining sample of plasmid extracted this day (20μl) : samples 3, 4 and 6
| component | volume |
|---|---|
| Plasmid | 20 μl |
| Fast Digest buffer 10X | 3 μl |
| EcoRI | 1μl |
| PstI | 1μl |
| H20 | 5 μl |
37°C - at night
Bacterial culture of pGEMTeasy+chromoprotein samples 3,4 and 6 from bacterial stock
by Laetitia
Culture in 5mL LB + ampiciline (1/1000) - 37°C at night
D - Lemon Scent
Streaking of colonies transformed by BBa_K762100+pGEMTeasy (LS)
Due to yesterday's strange results, we conduct 30 streakings in a petri dish: 28 white colonies and 2 blue colonies.
Digestion of pGEMTeasy+ LS by SacI
by Laetitia
The goal is to check if the limonen synthase gene is really inside the plasmid. We used the two samples named " LS2 " and " LS3 " which are normally pGEMTeasy+LS .
| component | volume |
|---|---|
| Plasmid | 20 μl |
| Fast Digest buffer 10X | 3μl |
| SacI | 1μl |
| H20 | 6μl |
Electrophoresis of the digestion products by SacI
by Laetitia
T : control negatif without enzyme
| component | volume |
|---|---|
| blue | 2 μl |
| Plasmid | 2 μl |
| H20 | 8μl |
D : sample digested by SacI
L - TLS2 - DLS2 - TLS3 - DLS3
(photo)
The results are weird, the bands on the gel don't match with our expectation, we don't know what happened with this plasmid. We should see two bands because if the limonene synthase gene were inside the plasmid but we only can see one band which doesn't match with the size of pGEMTeasy+LS or with pGEMTeasy empty...
 "
"