Team:Paris Saclay/Project/Lemon Scent
From 2014.igem.org

Contents |
Lemon Scent
Countdown
This page is under Melanie's responsibility
- Deadline: 12/oct
- Final review by Sylvie.
Summary
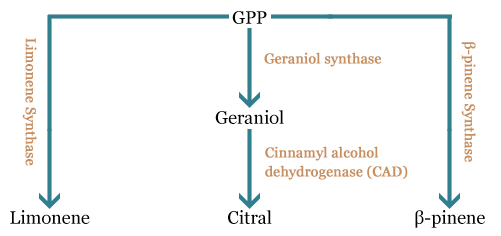
In the part of the project, we want to express the main components of the lemon scent (limonene, β-pinene and citral) in the odor-free E. coli we constructed. For this, we plan to engineer a plasmid constructed by the team of Taek Soon Lee, bearing a complete mevalonate pathway, a geranyldiphosphate synthase and a (S)-limonene synthase, to express (i) a β-pinene synthase (BBa_K517003), (ii) a geraniol synthase (GES) and a cinnamyl alcohol dehydrogenase (CAD) to produce β-pinene and citral respectively. We also would like to characterize the limonene synthase biobrick constructed by the Edinburgh team (BBa_K762100) and verify whether it is functional by replacing the intial (S)-limonene synthase by the biobrick.
We managed to construct two plasmids, the plamsid containing the β-pinene synthase gene and the plasmid containing the geraniol synthase gene. Due to lack of time, we could not obtain the other plasmids.
Introduction
The lemon scent is due to many volatile compounds, but the three main components are the (S)-limonene, β-pinene and citral molecules. All these molecules belong to the terpene family of metabolites.[1][6]
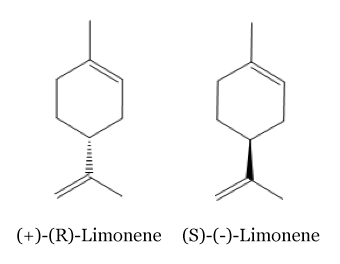
Limonene
There are two isomeric forms of the limonene molecules: Odor of these two isomers are different: the (R)-Limonene has a orangey smell whereas the (S)-Limonene has a strongly lemon scent. [2]
Pinene
There are also two isomeric forms of pinene: α- and β-pinene. In the lemon oil, β-pinene represents between 10-17% of the total compounds [3]. In contrast, there is no trace of α-pinene.
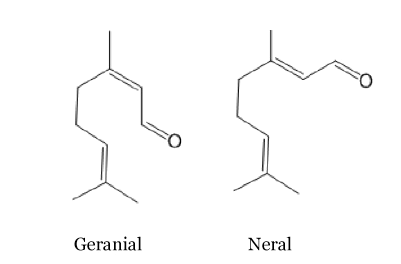
Citral
This is the principal component of the lemon grass and lemon smell and it is present in many other plants such as orange, verbena... Here again there are two stereoisomeric forms, geranial and neral. The trans isomer, geranial or citral A, has a strong lemon scent. Whereas, the cis isomer named neral or citral B has sweaker lemon scent. These compounds are often used in the perfume industry to reproduce the smell of lemon.
All these compounds come from a common precursor, the geranylpyrophosphate (GPP). This precursor is converted in one or two steps in the molecules we are interested in obtaining.
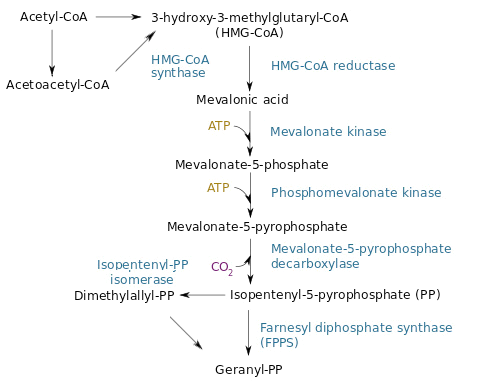
The desoxyxylulose 5-phosphate (DXP) parthway is the common pathway used by prokaryote cells to produce GPP. E. coli synthesizes GPP using the DXP pathway but in low quantity [4]. However, another pathway called the mevalonate pathway (MEV) exists in eukaryote and some prokaryotes. Moreover, plants can use both pathways to produce GPP in higher quantity. To copy the plants' strategy, we chose to add the MEV pathway to our engineered E. coli in order to produce higher quantity of GPP and thereby obtain a stronger lemon smell. The figure below is a scheme of the MEV pathway:
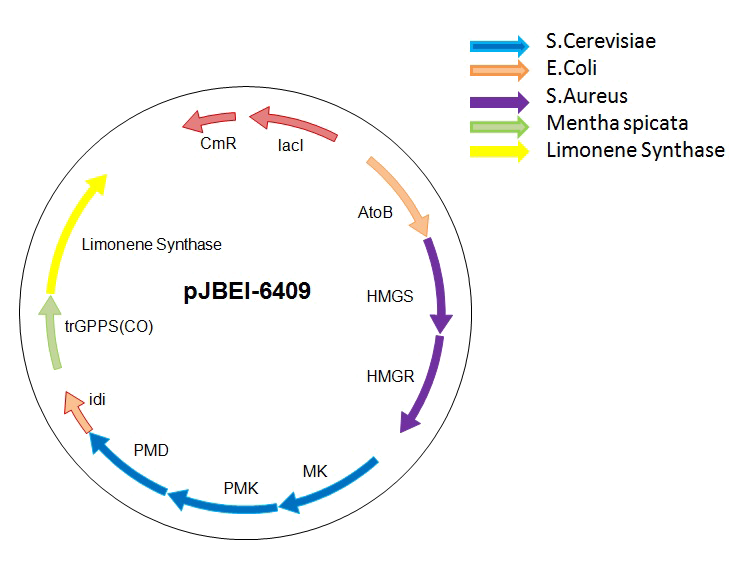
To tackle the problem of the low GPP bioavailability in E. coli, the Taek Soon Lee’s research group designed and constructed a plasmid with a complete biosynthetic mevalonate pathway for the production of GPP [3]. Then they expressed the Abies grandis (S)-limonene synthase (LS) gene in the same plasmid, called pJBEI6409. With this construction, they were able to produce 400 mg/L of (S)-limonene.
We decided to use their construction for the production of (R)-limonene, β-pinene and citral in E. coli. We thank the team of Taek Soon Lee for the gift of the pJBEI6409 plasmid.
Principle of our Constructions
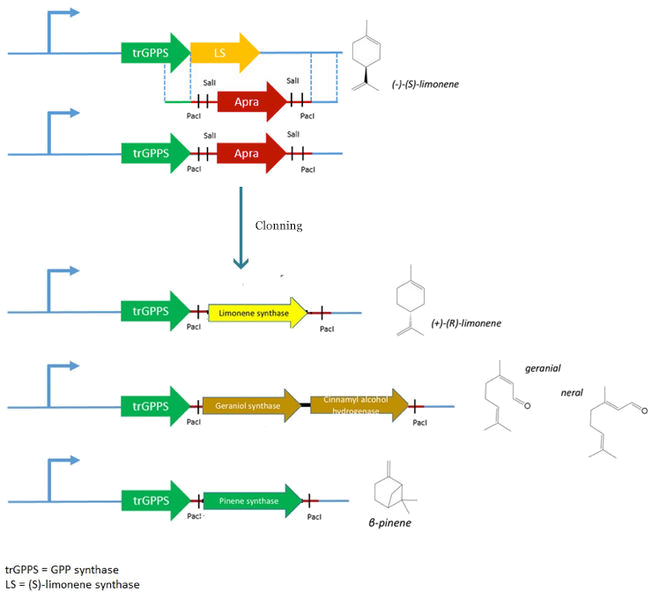
For our project, we need to replace the (S)-limonene synthase (LS) gene by the (R)-limonene synthase gene (BBa_K762100), the β-pinene synthase gene (BBa_K517003) and the geraniol synthase (GES)- cinnamyl alcohol dehydrogenase (CAD) genes.
However, pJBEI6409 was constructed using a 3A assembly method, meaning that there are no restriction sites flanking the LS gene. We therefore decided to replace the (S)-LS gene by an apramycin resistance cassette flanked by PacI and SalI restriction sites by double homologous recombination. The resulting plasmid (pPS1) will then be used to clone the various genes (limonene synthase – β-pinene synthase – geraniol synthase and cinnamyl alcohol dehydrogenase).
Construction of pPS1
Replacement of the LS gene by an apramycin cassette
The first step of this sub-project is to replace the (S)-limonene synthase (LS) gene by an apramycin-resistant cassette. This cassette is flanked by PacI and SalI restriction sites to facilitate the cloning of the different genes.
The (S)-LS starting gene is replaced by the apramycin cassette by PCR targeting.
- PCR amplification of the apramycin cassette with oligonucleotides iPS70 and iPS71 that contain the restriction sites PacI and SalI. The extremities of these oligonucleotides are homologous with the sequence that constitutes the LS gene.
- Transformation of the E. coli DY330 strain [5] (hyperrecombinant strain) by the pJBEI6409 plasmid.
- Transformation of the E. coli DY330/pJBEI6409 with the PCR product after induction of the recombination genes.
- Verification of the clones by BglII digestion.
Construction pPS2
Replacement of the apramycin cassette by the (R)- LS gene, Bba_K762100
This step consists in replacing the apramycin resistance gene cassette introduced in pJBEI6409 by the (R)-LS gene.
- PCR amplification of Bba_K762100 with oligonucleotides iPS66 and iPS67 that contain the restriction sites PacI and SalI.
- TA-cloning of the PCR product in the Pcr 4 Blunt-Topo vector (LS Topo plasmid).
- Verification of the clones LS-TOPO by PCR with the oligonucleotides iPS66 and iPS67 and sequencing.
- Digestion of the LS-TOPO plasmid by PacI and by MscI to eliminate the topo vector.
- Digestion of pPS1 by PacI.
- Ligation of the LS gene into pPS1/PacI.
- Transformation of E. coli DH5α with the ligation product.
- Verification of the clones by PCR with the oligonucleotides iPS72 and iPS96
Here we failed to obtain an insert in the correct orientation.
Construction of pPS3
Replacement of the apramycin cassette by the β pinene gene, BBa_K517003
This step consists in replacing the apramycin resistance gene cassette introduced in pJBEI6409 by the β pinene gene.
- PCR amplification of Bba_K517003 with oligonucleotides iPS68bis and iPS69 that contain the restriction sites PacI and SalI.
- TA-cloning of the PCR product in the Pcr 4 Blunt-Topo vector (PS Topo plasmid).
- Verification of the clones PS-TOPO by PCR with the oligonucleotides iPS68bis and iPS69 and sequencing.
- Digestion of the PS-TOPO plasmid by PacI and by PstI to eliminate the topo vector.
- Digestion of pPS1 by PacI.
- Ligation of the PS gene into pPS1/PacI.
- Transformation of E. coli DH5α with the ligation product.
- Verification of the clones by PCR with the oligonucleotides iPS72 and iPS93.
Construction of pPS4
Replacement of the apramycin cassette by the geraniol synthase gene,
This step consists in replacing the apramycin resistance gene cassette introduced in pJBEI6409 by geraniol synthase gene -(GS) (obtained from Marc Fischer, from the Grapevine Secondary Metabolism team of the ‘grapevine health and wine quality' research unit (Colmar)).
- PCR amplification of the gene (pcola plasmid) with oligonucleotides iPS81bis and iPS82 that contain the restriction sites PacI and SalI.
- TA-cloning of the PCR product in the Pcr 4 Blunt-Topo vector (GS Topo plasmid).
- Verification of the clones GS-TOPO by PCR with the oligonucleotides iPS81bis and iPS82 and sequencing.
- Digestion of the GS-TOPO plasmid by PacI and MscI to eliminate the topo vector.
- Digestion of pPS1 by PacI.
- Ligation of the GS gene into pPS1/PacI.
- Transformation of E. coli DH5α with the ligation product.
- Verification of the clones by PCR with the oligonucleotides iPS72 and iPS96
Construction of pPS5
Cloning of the CAD gene in pPS4
This step consists in cloning the Cinnamyl alcohol deshydrogenase (CAD) gene in the SalI site of pPS4
- PCR amplification of the truncated CAD gene (synthetic gene with oligonucleotides iPS79 and iPS80 that contain the restriction sites PacI and SalI).
- TA-cloning of the PCR product in the PCR 4 Blunt-Topo vector (CAD Topo plasmid).
- Verification of the clones CAD-TOPO by PCR with the oligonucleotides iPS79 and iPS80 and sequencing.
- Digestion of the CAD-TOPO plasmid by SalI and PvuII to eliminate the topo vector.
- Digestion of pPS4 by SalI.
- Ligation of the CAD gene into pPS4/SalI.
- Transformation of E. coli DH5α with the ligation product
- Verification of the clones by PCR
References
- [1] Marcelo Carnier Dornelas - A genomic approach to characterization of the Citrus terpene synthase gene family - Genet. Mol. Biol. vol.30 no.3 suppl.0 São Paulo 2007
- [2] http://www.societechimiquedefrance.fr/produit-du-jour/limonene-et-monoterpenes.html
- [3] http://booksofdante.wordpress.com/tag/beta-pinene/
- [4] Jorge Alonso-Gutierrez and al - Metabolic engineering of Escherichia coli for limonene and perillyl alcohol production – metabolique engineering 2013
- [5] Daiguan Yu and al - An efficient recombination system for chromosome engineering in Escherichia coli – 2000)
- [6] http://www.aromaticplantproject.com/articles_archive/citrus_essential_oils.html
 "
"