Team:Paris Saclay/Notebook/August/27
From 2014.igem.org
(→Collection of pellets containing pGEMTeasy+chromoprotein) |
(→Lab Work) |
||
| Line 9: | Line 9: | ||
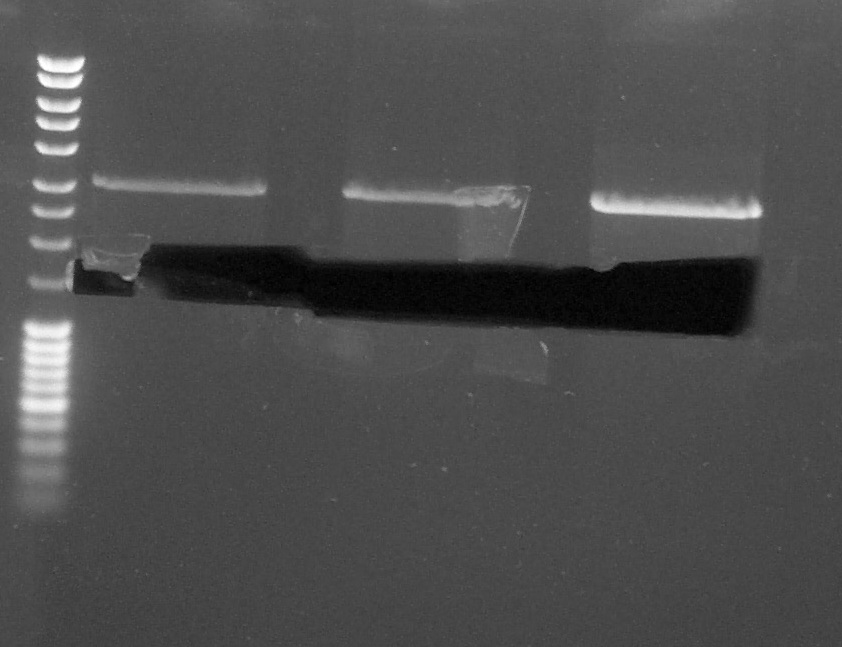
Digestion made the 26th by Laetitia. After the migration on agarose gel (100V), we cut the band corresponding to the chromoprotein gene on UV table. | Digestion made the 26th by Laetitia. After the migration on agarose gel (100V), we cut the band corresponding to the chromoprotein gene on UV table. | ||
| + | [[File:270814 Laetitia gel decoup chromo.jpg|300px]] | ||
====Purification of the chromoprotein gene from the agarose gel ==== | ====Purification of the chromoprotein gene from the agarose gel ==== | ||
| Line 14: | Line 15: | ||
We used the kit PCR-Clean-UP to extract the DNA from the agarose gel. Elution volume : 15µl | We used the kit PCR-Clean-UP to extract the DNA from the agarose gel. Elution volume : 15µl | ||
| - | |||
====Gel electrophoresis of the purification product ==== | ====Gel electrophoresis of the purification product ==== | ||
Revision as of 15:30, 28 August 2014
Wednesday 27th August
Lab Work
B - Construction of the fusion protein (color)
Electrophoresis of pGEMTeasy+chromoprotein 3,4 and 6 by EcoRI and PstI
by Laetitia
Digestion made the 26th by Laetitia. After the migration on agarose gel (100V), we cut the band corresponding to the chromoprotein gene on UV table.
Purification of the chromoprotein gene from the agarose gel
by Sean
We used the kit PCR-Clean-UP to extract the DNA from the agarose gel. Elution volume : 15µl
Gel electrophoresis of the purification product
by Sean
The goal is to check if the purification of the chromoprotein gene has worked.
PCR clean-up was performed ealier today. 1µl of each result was used for the electrophoresis. Nothing appeared under UV.
Ligation of chromoprotein
by Sean
| components | volumes |
|---|---|
| H2O | 6μl |
| T4 DNA ligase buffer | 2µl |
| purified chromoprotein | 10µl |
| ligase | 1µl |
| pSB1C3 | 1µl |
Leave overnight at 16°C.
Collection of pellets containing pGEMTeasy+chromoprotein
by Sean
Three liquid cultures (pGEM+chromoprotein) were prepared. 2ml from each were collected in 2ml microcentrifuge tubes, then centrifuged. The pellets were put in the freezer.
Transformation of odor free E. coli with plasmids coding Fluo Protein
by Terry
Our fusion protein was not expressed in pGEMTeasy (no color in the culture), so, if our system do not work at all, I'm preparing FP (Fluorescent Protein) for our lemon.
6 plamids coding FP have been transformed in Odor Free :
- pEYFP
- pGFP
- pESFP
- pRFP+
- pCFP+
- pYFP+
D - lemon scent
Plasmide extraction
By Mélanie
Extraction of pPS5 with the phenol protocol (as previously described)
PCR of Limonene synthase (BBa762100)
by Mélanie
5 tubes to do a gradient
| components | volumes |
|---|---|
| H2O | 29.75μl |
| Gotaq buffer | 10µl |
| dNTP 10mM | 1µl |
| iPS67 | 1µM |
| iPS66 | 1µM |
| DNA | 1µl |
| MgCl2 | 4µl |
| Gotaq | 1.25µl |
| step | temperature (°C) | time |
|---|---|---|
| 1 | 95 | 2min |
| 2 | 95 | 30 sec |
| 3 | 49-55 (gradient) | 30sec |
| 4 | 72 | 2min |
| 5 | 72 | 5min |
Electrophoresis of pPS3 and pPS4
by Terry
Result from yesterday 's extraction.
Alice, donne moi la photo.
 "
"