Team:Paris Saclay/Notebook/August/20
From 2014.igem.org
(→D- Lemon scent) |
(→D- Lemon scent) |
||
| Line 73: | Line 73: | ||
After the migration, we want to purify the band corresponding of pPS1 digested by Pac1. While exposing the gel to UV, we will cut the band and save it inside an Eppendorf, after the purification | After the migration, we want to purify the band corresponding of pPS1 digested by Pac1. While exposing the gel to UV, we will cut the band and save it inside an Eppendorf, after the purification | ||
| + | |||
| + | |||
| + | === Electrophoresis of pPSI === | ||
| + | We make an electrophoresis of pPSI to verify if the digestion made 19/08 has worked. It is usefull before starting the purification of the plasmid. | ||
| + | |||
| + | === Digestion of CAD and PMCS5 === | ||
| + | |||
| + | {| class="wikitable centre" width="50%" | ||
| + | |+ | ||
| + | |- | ||
| + | ! scope=col | components | ||
| + | ! scope=col | volumes | ||
| + | |- | ||
| + | |CAD | ||
| + | |5 μl | ||
| + | |- | ||
| + | |10x buffer fastDigest | ||
| + | |1 μl | ||
| + | |- | ||
| + | |PacI | ||
| + | |0.5 µl | ||
| + | |- | ||
| + | |H<sub>2</sub>O | ||
| + | |3.5 µl | ||
| + | |} | ||
| + | |||
| + | |||
| + | {| class="wikitable centre" width="50%" | ||
| + | |+ | ||
| + | |- | ||
| + | ! scope=col | components | ||
| + | ! scope=col | volumes | ||
| + | |- | ||
| + | |PMCS5 | ||
| + | |15 μl | ||
| + | |- | ||
| + | |10x buffer fastDigest | ||
| + | |2 μl | ||
| + | |- | ||
| + | |PacI | ||
| + | |0.5 µl | ||
| + | |- | ||
| + | |H<sub>2</sub>O | ||
| + | |2.5 µl | ||
| + | |} | ||
| + | |||
| + | |||
| + | |||
====B - Construction of the fusion protein (color)===== | ====B - Construction of the fusion protein (color)===== | ||
Revision as of 16:10, 20 August 2014
Contents |
Wednesday 20th August
D- Lemon scent
Bacterial culture
by Laetitia
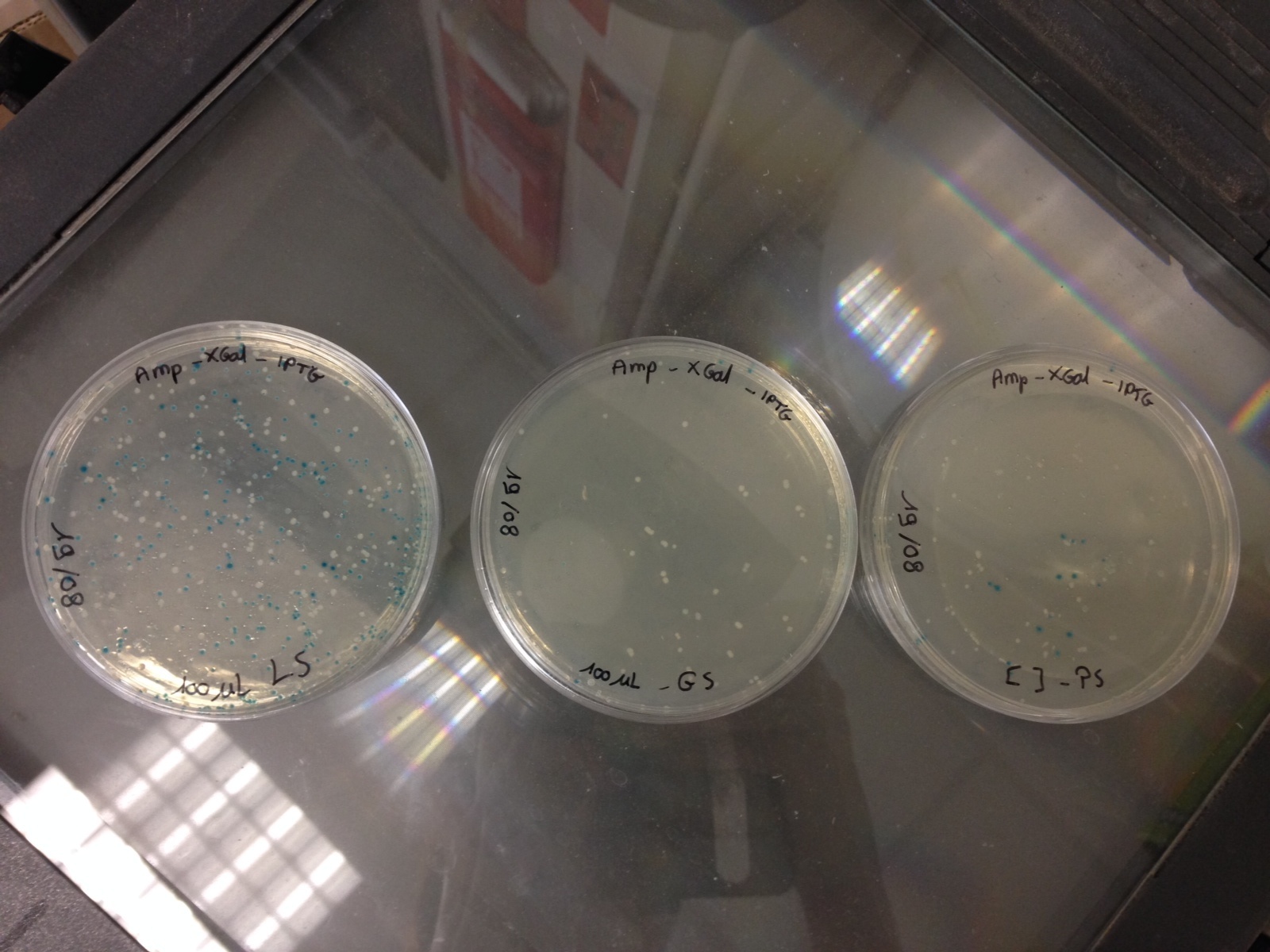
Here are the results of the bacterial culture of yesterday
Bacteria with the pGEMTeasy without the DNA insert are blue and the bacteria with the pGEMTeasy and the DNA insert (PS, GS or LS gene) are white
Then, we have chosen 3 white colonies for each DNA insert and we have launched 3 corresponding liquid cultures :
- 5mL LB + ampiciline (1/1000e)
- 1 white colony
The 9 cultures are incubated at 37°C
by Melanie
Preculture 20ml of DH5a pPSI (apra 1/2000)
CAD PCR
Today we have receive our synthetic gene CAD (cinnamyl alcohol deshydrogenase) :)
so we do a PCR (with go taq):
23.25 µl --> H2O
10 µl --> 5xbuffer
1 µl --> dNTP
4 µl --> MgCl2
5 µl iPS 79 (Primer)
5 µl iPS 80
Very small quantity of DNA
1.5 µl --> DMSO
0.25 µl --> Gotaq
PCR cycle
95° 2 min 95° 30 sec 58° 30 sec 72° 1 min 45 72° 5 min
Migration of pPS1 digested by PacI
by Huang vu and Laetitia
pPS1 previously digested by PacI is filled inside an agarose gel :
-1g agarose
-1mL TAE
-2 µL BET
The totality of the sample is filled (around 38µL) and we filled 20µl of ladder. Migration 100V
After the migration, we want to purify the band corresponding of pPS1 digested by Pac1. While exposing the gel to UV, we will cut the band and save it inside an Eppendorf, after the purification
Electrophoresis of pPSI
We make an electrophoresis of pPSI to verify if the digestion made 19/08 has worked. It is usefull before starting the purification of the plasmid.
Digestion of CAD and PMCS5
| components | volumes |
|---|---|
| CAD | 5 μl |
| 10x buffer fastDigest | 1 μl |
| PacI | 0.5 µl |
| H2O | 3.5 µl |
| components | volumes |
|---|---|
| PMCS5 | 15 μl |
| 10x buffer fastDigest | 2 μl |
| PacI | 0.5 µl |
| H2O | 2.5 µl |
B - Construction of the fusion protein (color)=
PCR with Gotaq
22.75 µl --> H2O
10 µl --> 5xbuffer
1 µl --> dNTP
4 µl --> MgCl2
5 µl iPS 83 (Primer)
5 µl iPS 84
0.5 µl --> DNA
1.5 µl --> DMSO
0.25 µl --> Gotaq
PCR cycle
95° 2 min 95° 30 sec 58° 30 sec 72° 1 min 45 72° 5 min
 "
"