Team:Paris Saclay/Modeling/odor
From 2014.igem.org
(→Modeling) |
(→Modeling) |
||
| Line 45: | Line 45: | ||
| - | + | * We have total reaction and it exist a time where $[S] = 0$. It imply that $V = 0$. | |
| - | + | * We reach a steady state between the formation and the destruction of the product. We have also $V = 0$. | |
In fact we can consider that the speed of reaction decrease to 0. It will allow us to get an expression of $V_0$ in function of known parameters. But we must add others experimentals conditions. We consider the conditions set by Michaelis. | In fact we can consider that the speed of reaction decrease to 0. It will allow us to get an expression of $V_0$ in function of known parameters. But we must add others experimentals conditions. We consider the conditions set by Michaelis. | ||
| Line 143: | Line 143: | ||
where $Vm_f$ and $Km_f$ are constant relative to the Fragrance ie Limonene, Geraniol or Pinene. | where $Vm_f$ and $Km_f$ are constant relative to the Fragrance ie Limonene, Geraniol or Pinene. | ||
| - | To solve these equation, we use the logiciel R and we obtain these following results for the production of Limonene: | + | To solve these equation, we use the logiciel R and we obtain these following results for the production of Limonene, geraniol and pinene: |
The differents constant are obtained via Brenda enzyme database: http://www.brenda-enzymes.org/ | The differents constant are obtained via Brenda enzyme database: http://www.brenda-enzymes.org/ | ||
| Line 150: | Line 150: | ||
[[File:Paris_Saclay_odor_1.jpg|700px]] | [[File:Paris_Saclay_odor_1.jpg|700px]] | ||
| - | [[File:Paris_Saclay_odor_60.jpg| | + | [[File:Paris_Saclay_odor_60.jpg|700px]] |
| - | [[File:Paris_Saclay_odor_6000.jpg| | + | [[File:Paris_Saclay_odor_6000.jpg|700px]] |
| - | [[File:Paris_Saclay_odor_60000.jpg| | + | [[File:Paris_Saclay_odor_60000.jpg|700px]] |
Revision as of 12:49, 5 October 2014

Contents |
Modeling
Countdown
This page is under Eugene's responsibility
- Deadline: 08/oct.
- Final text
- Deadline: 12/oct
- Final review by Claire.
Odor
To synthesize the smell of lemon, we have to mainly produce Limonene, Geraniol and Pinene. The combination of these products will allow us to get the fragrance we need. We propose here a study of the evolution of these concentrations over time by using the technique Michaelis-Menten proposed in 1913 by Leonor Michaelis and Maud Menten. This will allow us to obtain a description of the kinetics of reactions between an enzyme (biological catalyst) and a substrate for giving a product.
Basic model
We consider the reations below between the enzyme $E$ and the substrate $S$. $ES$ is an complex foregoing the formation of the product P.
\[ E + S {\rightarrow}^{k_1} ES {\rightarrow}^{k_2} E + P \]
\[ E + S {\leftarrow}_{k_{-1}} ES {\leftarrow}_{k_{-2}} E + P \]
where
\[ \left\lbrace \begin{array}{llll} k_1 \text{ is the association constant of } E \text{ and } S \\ k_{-1} \text{ is the dissociation constant of } ES \\ k_2 \text{ is the reaction constant of } ES \text{ in } E + P \\ k_{-2}\text{ is the association constant of } E \text{ and } P \\ \end{array} \right. \]
We want to model the kinetics of the reaction catalyzed by enzymes Michaelis. We are interested in the concentration of molecules and various constants that we have described above.
The speed of the reaction $V$ is caracterised by the speed of apparition of the product. It is given by $V = \frac{d[P]}{dt}$ where $[P]$ is the concentration of the product. Modeling phenomena are inextricably linked to the reality of the phenomenon modeled, so we must take into account a number of experimental facts.
- At the beginning, we don't have a product $P$. So the reaction $ES {\leftarrow}_{k_{-2}} E + P$ doesn't exist.
- The speed of the reaction is constant equal to $V_0$. It only depend to the initiale concentration of substrate $[S]_0$ and enzyme $[E]_T$.
- Over time, the concentration of the substrate decrease while the concentration of P increase. We have only two possibles cases:
* We have total reaction and it exist a time where $[S] = 0$. It imply that $V = 0$. * We reach a steady state between the formation and the destruction of the product. We have also $V = 0$.
In fact we can consider that the speed of reaction decrease to 0. It will allow us to get an expression of $V_0$ in function of known parameters. But we must add others experimentals conditions. We consider the conditions set by Michaelis.
Michaelis condition
To simplify the problem, Michaelis consider that:
- the concentration of substrate is very large relative to the concentration of enzyme: $[S] >> [E]_T $ and the concentration of the product is negligible: $P \approx 0$. With the equation $ES {\leftarrow}_{k_{-2}} E + P$, we know that the speed formation of $ES$ is gived by $v = k_{-2}[E][P] \approx 0$.
- We suppose that we reach the equilibrium between $[E], [S]$ and $[ES]$ fastly. In the case of equilibrium, $[ES]$ is constant until $[P] \approx 0$, and so
$\frac{d[ES]}{dt} = 0$.
- $[S]_0 >> [E]_T \rightarrow [ES]_{max} << [S]_0$ because the concentration of the complex $[ES]$ is limited by the concentration of enzyme.
Since $[S] = [S]_0 - [ES]$ and $[ES] \approx 0$, we have $[S] = [S]_0$.
In the following, we will stay in the conditions of Michaelis.
$\forall t, V = \frac{d[P]}{dt} = k_2 [ES] - k_{-2}[E][P]$. Since initially $[P] \approx 0$, we have $\boxed{V_i = k_2 [ES]}$. The problem is that $[ES]$ is unknow ! \newline
The variation of the concentration of the complexe $ES$ is given by the difference between it formation and it dissociation. This imply
\begin{align*} % & \frac{d[ES]}{dt} = k_1 [E][S ] - (k_{-1} + k_2) [ES] = 0 \text{ in initial condition }. \\ & \text{ So, } \frac{[E][S]}{[ES]} = \frac{k_{-1} + k_2}{k1} =: Km = \text{ Michaelis constant }. \\ & \text{ We deduce the Michaelis equation } \boxed{[ES] = \frac{[E][S]}{Km}}. % \end{align*}
For all time, the total enzyme $E$ is composed by free molecule of $E$ and molecule associated to the substrate to form $ES$. It is expressed by $\forall t, [E]_T = [E] + [ES]$, where we deduce $[E] = [E]_T - [ES]$. Replacing this in the Michaelis equation, we have :
\begin{align*} % & [ES] = \frac{([E]_T - [ES])[S]}{Km} = \frac{[E]_T [S]}{Km} - \frac{[ES][S]}{Km}. \\ & [ES](1 + \frac{[S]}{Km}) = \frac{[E]_T [S]}{Km} \\ & [ES] = \frac{\frac{[E]_T [S]}{Km} }{ \frac{Km + [S]}{Km} } = [E]_T \times \frac{[S]}{Km + [S]}. % \end{align*}
Returning to the expression of $V_i = k_2 [ES]$ and replacing $[ES]$ by the last expression below, we have $V_i = k_2 [E]_T \times \frac{[S]}{Km + [S]} $
\[ \text{Since } \left\lbrace \begin{array}{ll} [ES] \leq [E]_T \\ V_i = k_2 [ES] \end{array} \right. \text{ we have } \boxed{ V_{max} = k_2 [E]_T } (k_2 \text{ is also noted } k_{cat}). \]
Taking into account all these results, we obtain the following equation of Michaelis-Menten
\[ \boxed{ V_i = V_{max} \frac{[S]}{[S] + Km} } . \]
We can determine $V_{max}$ and $Km$ experimentally. $[S] \approx [S]_0$ is the concentration of substrate at the beginning.
In the following, we will work directly with this expression.
Biosynthetic pathway of limonene
Complete model
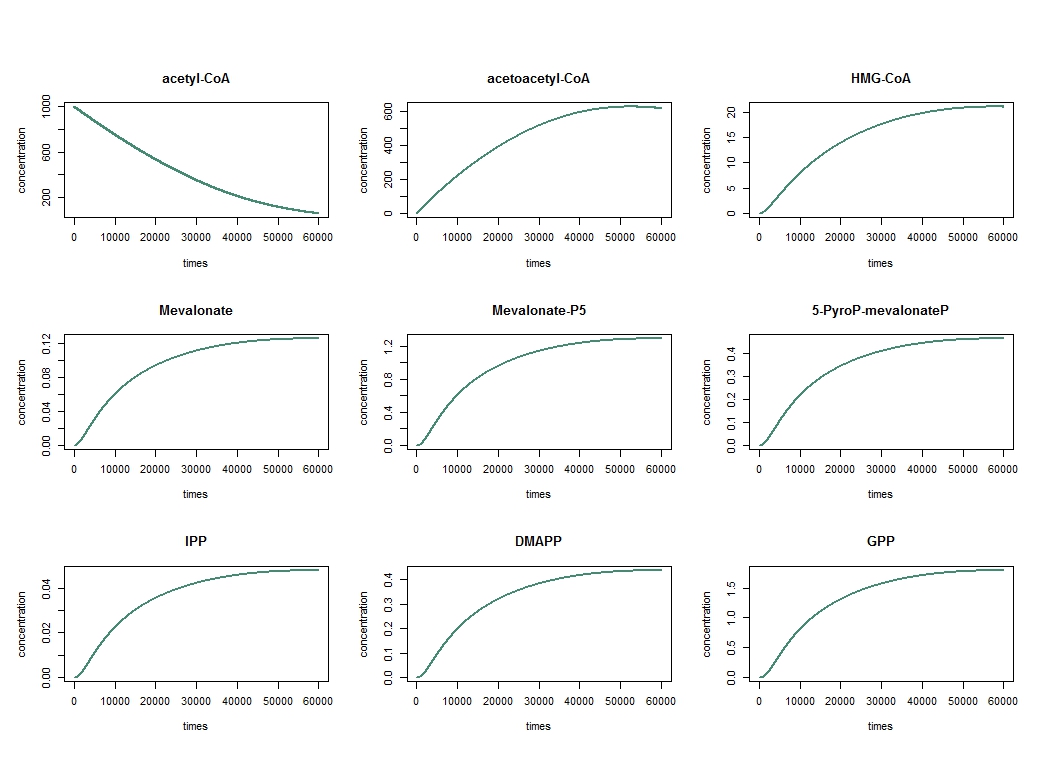
By analysing the different reaction in pathway above and using the equation of Michaelis-Menten, we obtain the system of nine equation below:
\[
\left\lbrace
\begin{array}{llllllllll}
\frac{d[acetyl-CoA]}{dt} &= - \frac{Vm1 [acetyl-CoA]}{Km1 + [acetyl-CoA]} \\
\frac{d[acetoacetyl-CoA]}{dt} &= \frac{Vm1 [acetyl-CoA]}{Km1 + [acetyl-CoA]} - \frac{Vm2 [acetoacetyl-CoA]}{Km2 + [acetoacetyl-CoA]} \\
\frac{d[HMG-CoA]}{dt} &= \frac{Vm2 [acetoacetyl-CoA]}{Km2 + [acetoacetyl-CoA]} - \frac{Vm3 [HMG-CoA]}{Km3 + [HMG-CoA]} \\
\frac{d[Mevalonate]}{dt} &= \frac{Vm3 [HMG-CoA]}{Km3 + [HMG-CoA]} - \frac{Vm4 [Mevalonate]}{Km4 + [Mevalonate]} \\
\frac{d[MevalonateP-5]}{dt} &= \frac{Vm4 [Mevalonate]}{Km4 + [Mevalonate]} - \frac{Vm5 [MevalonateP-5]}{Km5 + [MevalonateP-5]} \\
\frac{d[5-pyroP-MevalonateP]}{dt} &= \frac{Vm5 [MevalonateP-5]}{Km5 + [MevalonateP-5]} - \frac{Vm6 [5-pyroP-MevalonateP]}{Km6 + [5-pyroP-MevalonateP]} \\
\frac{d[IPP]}{dt} &= \frac{Vm6 \frac{[5-pyroP-MevalonateP]}{2}}{Km6 + \frac{[5-pyroP-MevalonateP]}{2}} - \frac{Vm7 \frac{[IPP]}{2}}{Km7 + \frac{[IPP]}{2}} - \frac{Vm8 \frac{[IPP]}{2}}{Km8 + \frac{[IPP]}{2}} \\
\frac{d[DMAPP]}{dt} &= \frac{Vm6 \frac{[5-pyroP-MevalonateP]}{2}}{Km6 + \frac{[5-pyroP-MevalonateP]}{2}} + \frac{Vm7 \frac{[IPP]}{2}}{Km7 + \frac{[IPP]}{2}} - \frac{Vm8 \frac{[DMAPP]}{2}}{Km8 + \frac{[DMAPP]}{2}} \\
\frac{d[GPP]}{dt} &= \frac{Vm7 \frac{[IPP]}{2}}{Km7 + \frac{[IPP]}{2}} + \frac{Vm8 \frac{[DMAPP]}{2}}{Km8 + \frac{[DMAPP]}{2}} - \frac{Vm_f [GPP]}{Km_f + [GPP]} \\
\frac{d[Fragrance]}{dt} &= \frac{Vm_f [GPP]}{Kmf + [GPP]}
\end{array}
\right.
\]
where $Vm_f$ and $Km_f$ are constant relative to the Fragrance ie Limonene, Geraniol or Pinene.
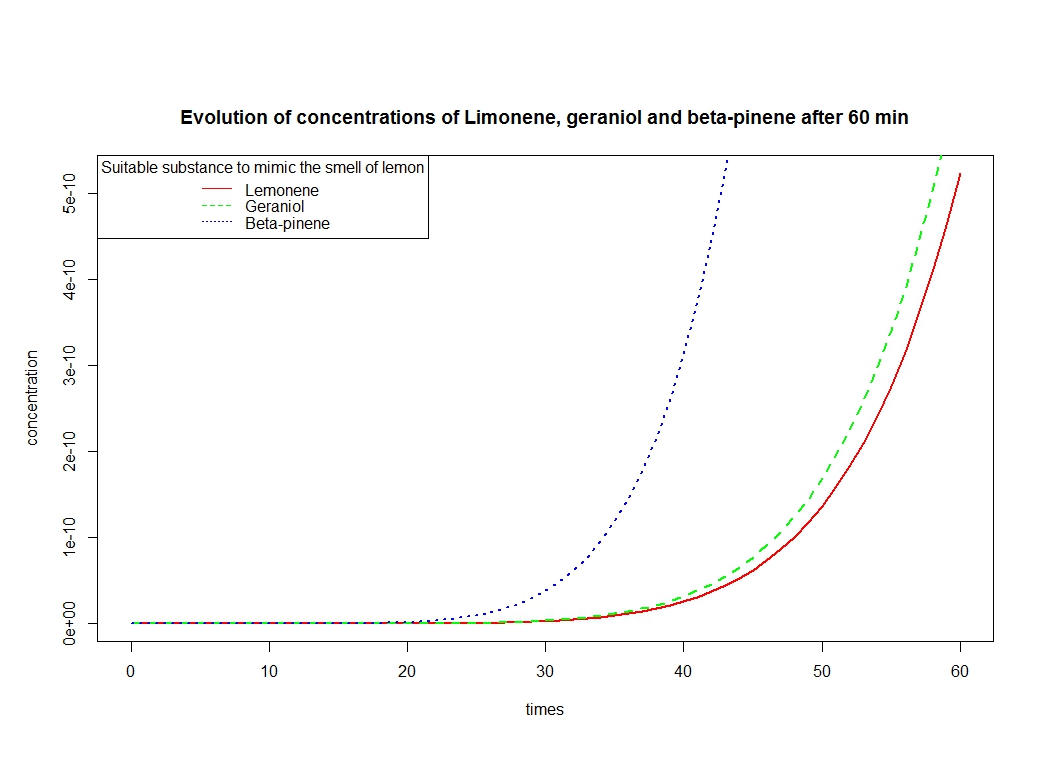
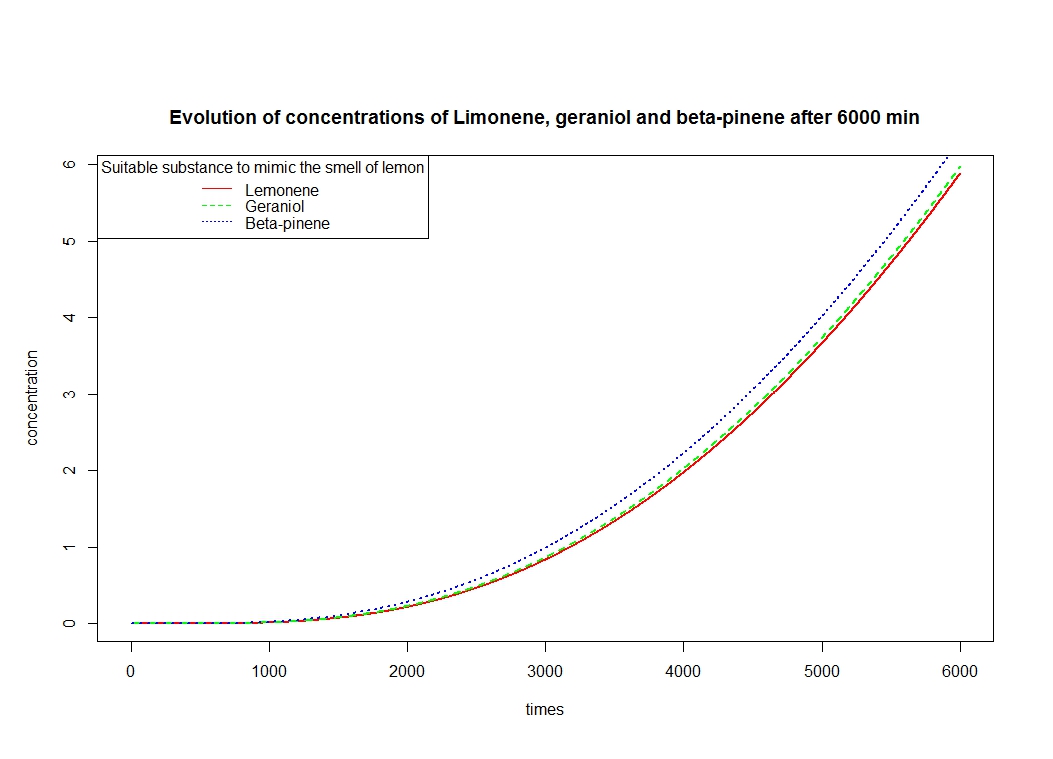
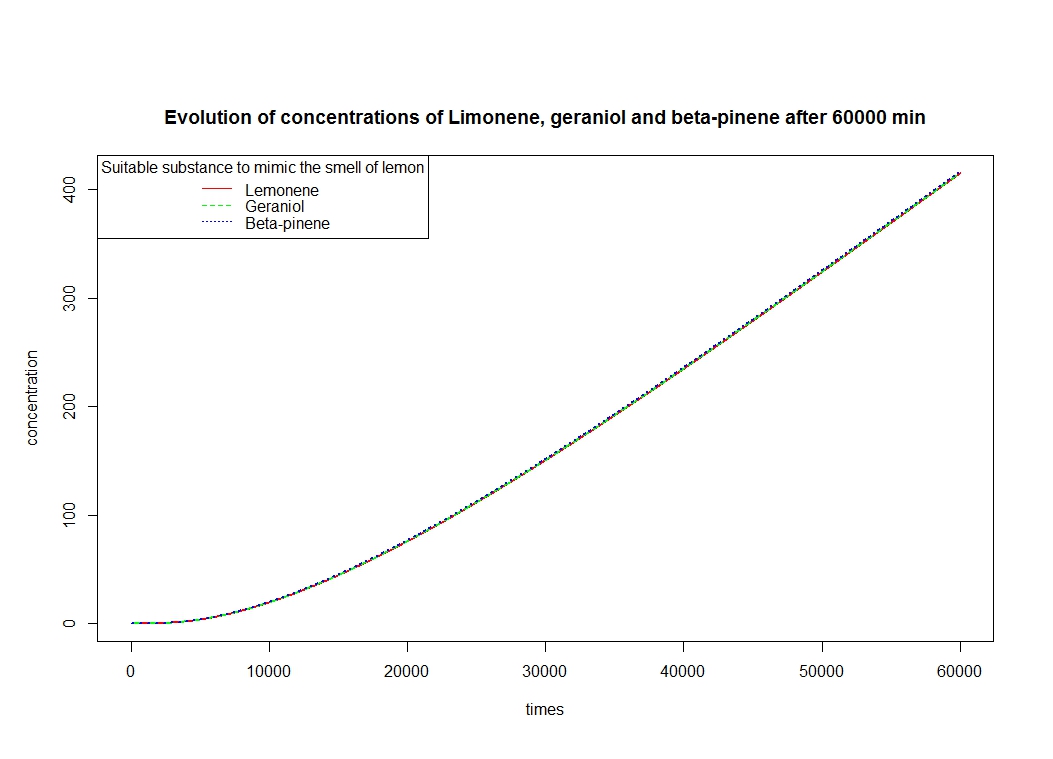
To solve these equation, we use the logiciel R and we obtain these following results for the production of Limonene, geraniol and pinene:
The differents constant are obtained via Brenda enzyme database: http://www.brenda-enzymes.org/
[3] Gilles Camus, La cinétique des enzymes michaeliennes et l'équation de Michaelis-Menten, 22 novembre 2012.
 "
"