Team:Paris Saclay/Notebook/August/19
From 2014.igem.org
(→Tuesday 19th August) |
m (→Bacterial culture) |
||
| (20 intermediate revisions not shown) | |||
| Line 2: | Line 2: | ||
=Tuesday 19th August= | =Tuesday 19th August= | ||
==Lab work== | ==Lab work== | ||
| + | |||
| + | ===Chromoprotein + Lemon scent=== | ||
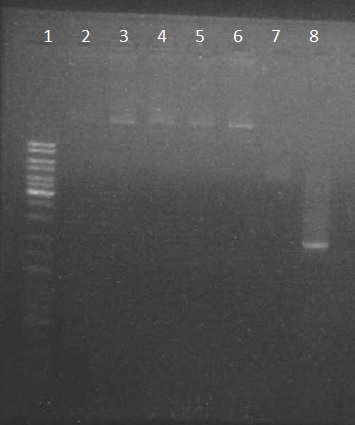
| + | Electrophoresis with the PpsI plasmide (obtain on thursday) + the chromoprotein PCR: | ||
| + | we obtain results as expected | ||
| + | |||
| + | #ladder 10µl | ||
| + | #pPSI 1.2µl | ||
| + | #pPSI 1.2µl | ||
| + | #pPSI 1.2µl | ||
| + | #pPSI 1.2µl | ||
| + | #pPSI 1.2µl | ||
| + | #p cola 1.2µl | ||
| + | #PCR chromoprotein 2µl | ||
| + | |||
| + | [[File:Paris_Saclay_140819.jpg]] | ||
| + | |||
| + | ===Lemon Scent=== | ||
| + | |||
| + | ==== Digestion of PCR results ==== | ||
| + | ''by Sean, Hoang Vu, Eugene'' | ||
| + | |||
| + | {| class="wikitable centre" width="50%" | ||
| + | |+ | ||
| + | |- | ||
| + | ! scope=col | components | ||
| + | ! scope=col | volumes | ||
| + | |- | ||
| + | |PCR purified LS | ||
| + | |15 μl | ||
| + | |- | ||
| + | |FastDigest green buffer 10X | ||
| + | |2 μl | ||
| + | |- | ||
| + | |PacI | ||
| + | |1 µl | ||
| + | |- | ||
| + | |H<sub>2</sub>O | ||
| + | |2 µl | ||
| + | |} | ||
| + | |||
| + | {| class="wikitable centre" width="50%" | ||
| + | |+ | ||
| + | |- | ||
| + | ! scope=col | components | ||
| + | ! scope=col | volumes | ||
| + | |- | ||
| + | |PCR purified GS | ||
| + | |15 μl | ||
| + | |- | ||
| + | |FastDigest green buffer 10X | ||
| + | |2 μl | ||
| + | |- | ||
| + | |PacI | ||
| + | |1 µl | ||
| + | |- | ||
| + | |H<sub>2</sub>O | ||
| + | |2 µl | ||
| + | |} | ||
| + | |||
| + | {| class="wikitable centre" width="50%" | ||
| + | |+ | ||
| + | |- | ||
| + | ! scope=col | components | ||
| + | ! scope=col | volumes | ||
| + | |- | ||
| + | |PCR purified PS | ||
| + | |15 μl | ||
| + | |- | ||
| + | |FastDigest green buffer 10X | ||
| + | |2 μl | ||
| + | |- | ||
| + | |PacI | ||
| + | |1 µl | ||
| + | |- | ||
| + | |H<sub>2</sub>O | ||
| + | |2 µl | ||
| + | |} | ||
| + | |||
| + | {| class="wikitable centre" width="50%" | ||
| + | |+ | ||
| + | |-''Italic text'' | ||
| + | ! scope=col | components | ||
| + | ! scope=col | volumes | ||
| + | |- | ||
| + | |Purified pPS1 | ||
| + | |30 μl | ||
| + | |- | ||
| + | |FastDigest green buffer 10X | ||
| + | |4 μl | ||
| + | |- | ||
| + | |PacI | ||
| + | |2 µl | ||
| + | |- | ||
| + | |H<sub>2</sub>O | ||
| + | |4 µl | ||
| + | |} | ||
| + | |||
| + | ====Purification of PCR products of limonen synthase==== | ||
| + | ''by Laetitia'' | ||
| + | |||
| + | We used the PCR products of limonen synthase prepared previously : [https://2014.igem.org/Team:Paris_Saclay/Notebook/August/18 August 18th] | ||
| + | |||
| + | The five samples were pooled in one and then purified using this protocol : [https://2014.igem.org/Team:Paris_Saclay/Protocols/PCR_clean-up protocol] | ||
| + | |||
| + | ====Bacterial transformation==== | ||
| + | ''by Laetitia'' | ||
| + | |||
| + | We used DH5alpha competent bacteria (prepared the 14/08) and they were transformed using the ligation product prepared previously [https://2014.igem.org/Team:Paris_Saclay/Notebook/August/18 August 18th] using this protocol : [https://2014.igem.org/Team:Paris_Saclay/Protocols/Transformation_of_supercompetent_Ecoli_cells_with_CaCl2 protocol] | ||
| + | |||
| + | We transformed 100µL of competent bacteria and we added the totality of the ligation product (pGEMTeasy containing LS or PS or GS) | ||
| + | |||
| + | ====Preparation of petri dishes==== | ||
| + | ''by Terry'' | ||
| + | |||
| + | {| class="wikitable centre" width="25%" | ||
| + | |+ 10 dishes with: | ||
| + | |- | ||
| + | ! scope=col | Antibiotic | ||
| + | ! scope=col | Concentration | ||
| + | |- | ||
| + | |XGal | ||
| + | |80μg/ml | ||
| + | |- | ||
| + | |IPTG | ||
| + | |2*10<sup>-3</sup> | ||
| + | |- | ||
| + | |Ampicillin | ||
| + | |10<sup>-3</sup> | ||
| + | |} | ||
| + | |||
| + | {| class="wikitable centre" width="25%" | ||
| + | |+ 4 dishes with: | ||
| + | |- | ||
| + | ! scope=col | Antibiotic | ||
| + | ! scope=col | Concentration | ||
| + | |- | ||
| + | |Ampicillin | ||
| + | |4*10<sup>-4</sup> | ||
| + | |} | ||
| + | |||
| + | ====Bacterial culture==== | ||
| + | ''by Laetitia'' | ||
| + | |||
| + | After the bacterial transformation, we spread for each strain (LS or PS or GS): | ||
| + | |||
| + | {| class="wikitable centre" width="25%" style="text-align:center" | ||
| + | |+ | ||
| + | |- | ||
| + | ! scope=col | dish type | ||
| + | ! scope=col | 100µl | ||
| + | ! scope=col | 200µl | ||
| + | ! scope=col | concentrate | ||
| + | ! scope=col | control | ||
| + | |- | ||
| + | |Ampicillin | ||
| + | |X | ||
| + | | | ||
| + | | | ||
| + | | | ||
| + | |- | ||
| + | |Ampicillin+IPTG+XGal | ||
| + | |X | ||
| + | |X | ||
| + | |X | ||
| + | |X | ||
| + | |} | ||
| + | |||
| + | We leave the dishes overnight at 37°C. | ||
| + | |||
| + | |||
| + | ==Photo of the Day== | ||
| + | [[File:Paris Saclay 19_august.jpg|600px|center]] | ||
| + | |||
| + | '''Members present:''' | ||
| + | * Instructors and advisors: Alice, Solenne and Sylvie. | ||
| + | * Students: Eugène, Hoang Vu, Laëtitia, Mélanie, Romain, Sean and Terry. | ||
| + | |||
{{Team:Paris_Saclay/notebook_footer}} | {{Team:Paris_Saclay/notebook_footer}} | ||
Latest revision as of 15:02, 14 October 2014
Contents |
Tuesday 19th August
Lab work
Chromoprotein + Lemon scent
Electrophoresis with the PpsI plasmide (obtain on thursday) + the chromoprotein PCR: we obtain results as expected
- ladder 10µl
- pPSI 1.2µl
- pPSI 1.2µl
- pPSI 1.2µl
- pPSI 1.2µl
- pPSI 1.2µl
- p cola 1.2µl
- PCR chromoprotein 2µl
Lemon Scent
Digestion of PCR results
by Sean, Hoang Vu, Eugene
| components | volumes |
|---|---|
| PCR purified LS | 15 μl |
| FastDigest green buffer 10X | 2 μl |
| PacI | 1 µl |
| H2O | 2 µl |
| components | volumes |
|---|---|
| PCR purified GS | 15 μl |
| FastDigest green buffer 10X | 2 μl |
| PacI | 1 µl |
| H2O | 2 µl |
| components | volumes |
|---|---|
| PCR purified PS | 15 μl |
| FastDigest green buffer 10X | 2 μl |
| PacI | 1 µl |
| H2O | 2 µl |
| components | volumes |
|---|---|
| Purified pPS1 | 30 μl |
| FastDigest green buffer 10X | 4 μl |
| PacI | 2 µl |
| H2O | 4 µl |
Purification of PCR products of limonen synthase
by Laetitia
We used the PCR products of limonen synthase prepared previously : August 18th
The five samples were pooled in one and then purified using this protocol : protocol
Bacterial transformation
by Laetitia
We used DH5alpha competent bacteria (prepared the 14/08) and they were transformed using the ligation product prepared previously August 18th using this protocol : protocol
We transformed 100µL of competent bacteria and we added the totality of the ligation product (pGEMTeasy containing LS or PS or GS)
Preparation of petri dishes
by Terry
| Antibiotic | Concentration |
|---|---|
| XGal | 80μg/ml |
| IPTG | 2*10-3 |
| Ampicillin | 10-3 |
| Antibiotic | Concentration |
|---|---|
| Ampicillin | 4*10-4 |
Bacterial culture
by Laetitia
After the bacterial transformation, we spread for each strain (LS or PS or GS):
| dish type | 100µl | 200µl | concentrate | control |
|---|---|---|---|---|
| Ampicillin | X | |||
| Ampicillin+IPTG+XGal | X | X | X | X |
We leave the dishes overnight at 37°C.
Photo of the Day
Members present:
- Instructors and advisors: Alice, Solenne and Sylvie.
- Students: Eugène, Hoang Vu, Laëtitia, Mélanie, Romain, Sean and Terry.
 "
"