Team:Paris Saclay/Notebook/August/22
From 2014.igem.org
(Difference between revisions)
m (→Friday 22nd August) |
m (→pPSI dephosphorylation) |
||
| (4 intermediate revisions not shown) | |||
| Line 25: | Line 25: | ||
====pPSI dephosphorylation==== | ====pPSI dephosphorylation==== | ||
| + | |||
| + | [[File:Paris_Saclay_140822.jpg|left]] | ||
After 2 hours, add 1.5µL of Alkaline Phosphatase (AP) and let it 1 hour. | After 2 hours, add 1.5µL of Alkaline Phosphatase (AP) and let it 1 hour. | ||
| Line 31: | Line 33: | ||
We've made a electrophoresis to check the digestion. | We've made a electrophoresis to check the digestion. | ||
| - | |||
'''Legend''' | '''Legend''' | ||
| Line 37: | Line 38: | ||
#pPSI+Apra digested via PacI, dephosphorylated 5µl | #pPSI+Apra digested via PacI, dephosphorylated 5µl | ||
#ladder 5µl | #ladder 5µl | ||
| - | |||
| - | |||
==== Ligation of BBa_K517003, BBa_K762100, and Geraniol synthase in pPSI==== | ==== Ligation of BBa_K517003, BBa_K762100, and Geraniol synthase in pPSI==== | ||
| Line 126: | Line 125: | ||
Finally put it in a incubator at 37°C for 30 minutes | Finally put it in a incubator at 37°C for 30 minutes | ||
| - | + | ||
====PCR of CAD ==== | ====PCR of CAD ==== | ||
| Line 163: | Line 162: | ||
{| class="wikitable centre" width="25%" | {| class="wikitable centre" width="25%" | ||
|+ kit PCR clean-up | |+ kit PCR clean-up | ||
| - | ! scope=col | | + | ! scope=col | DNA Polymerase |
| - | ! scope=col | | + | ! scope=col | volume |
|- | |- | ||
|Phusion | |Phusion | ||
| Line 193: | Line 192: | ||
|- | |- | ||
|} | |} | ||
| + | |||
| + | ==Photo of the Day== | ||
| + | [[File:Paris Saclay 22_august.jpg|600px|center]] | ||
| + | |||
| + | '''Members present:''' | ||
| + | * Instructors and advisors: Alice, Solenne and Sylvie. | ||
| + | * Students: Eugène, Hoang Vu, Laëtitia, Mélanie, Romain, Sean and Terry. | ||
| + | |||
| + | {{Team:Paris_Saclay/notebook_footer}} | ||
Latest revision as of 15:10, 14 October 2014
Contents |
Friday 22nd August
Lab work
Lemon Scent
pPSI digestion
| components | volumes |
|---|---|
| pPSI | 40μl |
| FastDigest green buffer 10X | 5μl |
| PacI | 2µl |
| H2O | 2µl |
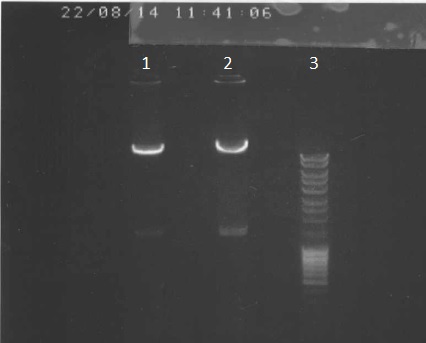
pPSI dephosphorylation
After 2 hours, add 1.5µL of Alkaline Phosphatase (AP) and let it 1 hour.
Then, inactivation of AP at 65°C during 15 minutes.
We've made a electrophoresis to check the digestion.
Legend
- pPSI+Apra digested via PacI, dephosphorylated 5µl
- pPSI+Apra digested via PacI, dephosphorylated 5µl
- ladder 5µl
Ligation of BBa_K517003, BBa_K762100, and Geraniol synthase in pPSI
| components | volumes |
|---|---|
| BBa_K517003 | 10μl |
| pPSI | 2μl |
| buffer | 2µl |
| H2O | 5µl |
| ligase | 1µl |
| components | volumes |
|---|---|
| BBa_K762100 | 10μl |
| pPSI | 2μl |
| buffer | 2µl |
| H2O | 5µl |
| ligase | 1µl |
| components | volumes |
|---|---|
| GS | 10μl |
| pPSI | 2μl |
| buffer | 2µl |
| H2O | 5µl |
| ligase | 1µl |
And let it in the freezer for 3 hours.
Transformation in competent E.coli DH5α
After 3 hours, take out the ligation tubes and add 100 µl of competent DH5α in each tube.
Then proceed to the transformation protocol.
- 20 minutes at 4°C
- 2 minutes 30 seconds at 42°C
- 2 minutes at 4 °C
- Add 0.9 ml of LB into the tubes
Finally put it in a incubator at 37°C for 30 minutes
PCR of CAD
With or synthetic gene, we decided to do a PCR to have more matrice.
| components | volumes |
|---|---|
| H2O | 27μl |
| 5x phusion buffer | 2µl |
| dNTP 10 mM | 2µl |
| iPS83 10µM | 5µl |
| iPS84 10µM | 5µl |
| DMSO | 1.5µl |
PCR purification
We use the kit PCR clean-up
the elution volume is 20µl
| DNA Polymerase | volume |
|---|---|
| Phusion | 0.5µl |
| Temperature (°C) | time |
|---|---|
| 98 | 30 sec |
| 98 | 10 sec |
| 58 | 30 sec |
| 72 | 45 sec |
| 72 | 10 min |
Photo of the Day
Members present:
- Instructors and advisors: Alice, Solenne and Sylvie.
- Students: Eugène, Hoang Vu, Laëtitia, Mélanie, Romain, Sean and Terry.
 "
"