Team:Paris Saclay/Notebook/August/8
From 2014.igem.org
Terry.Mara (Talk | contribs) (→Friday 8th August) |
m (→Photo of the Day) |
||
| (13 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
| + | {{Team:Paris_Saclay/notebook_header}} | ||
=Friday 8th August= | =Friday 8th August= | ||
==Lab work== | ==Lab work== | ||
| - | === | + | ===Salicylate Inducible Suppressing System=== |
| + | ====Transformation of competent E.coli cells==== | ||
| + | ''by Fabio'' | ||
| - | We | + | We transformed in DH5α the Ligation's result made [https://2014.igem.org/Team:Paris_Saclay/Notebook/August/7#Ligation yesterday] ('''BBa_K1372000''' with '''BBa_B0015'''). ''The samples were put at 37°C for 10 hours, then we put them at room temperature until monday morning)'' |
| - | + | '''Samples:''' | |
| + | # Sample 2 μl | ||
| + | # Sample 1 μl | ||
| + | # DH5α Control in LB | ||
| + | # DH5α Control in LB + Cm | ||
| + | '''Results on 11th August morning:''' | ||
| + | # Success, grown very well (> 200 colonies) | ||
| + | # Success, grown well (> 100 colonies) | ||
| + | # Success, a film of bacteria has settled | ||
| + | # Success, nothing has grown | ||
| + | [https://2014.igem.org/Team:Paris_Saclay/Protocols/Transformation_of_supercompetent_Ecoli_cells_with_CaCl2 Protocol] | ||
| + | ===Lemon Scent=== | ||
| + | ====PCR==== | ||
| + | ''by Mélanie & Pierre'' | ||
| + | We have done a PCR with pcola (geranyl synthase) and BBa_K517003: | ||
| - | MgCl2 = 4µl | + | * Green GotaqBuffer = 10µl |
| + | * MgCl2 = 4µl | ||
| + | * dNTP = 1µl | ||
| + | * Primer A = 1µM | ||
| + | * Primer B = 1µM | ||
| + | * DNA = 1µl | ||
| + | * Gotaq DNA polymerase = 0.25µl | ||
| - | + | Oligo used : iPS81bis AND iPS82 (pcola) iPS69 and iPS68bis (BBa_K517003) | |
| - | + | PCR cycles: | |
| - | + | * 95° 2min | |
| + | * 95° 30sec | ||
| + | * 49-55° 30 sec | ||
| + | * 72° 2min | ||
| + | * 71° 5min | ||
| - | + | ====Electrophoresis==== | |
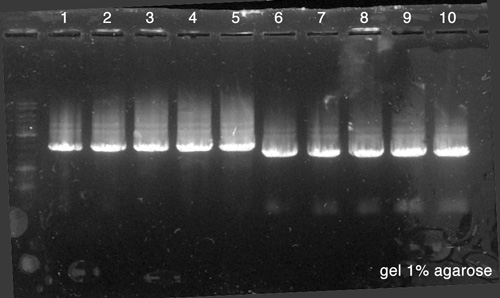
| + | [[File:Paris Saclay-080814-Pierre-Mélanie-PCR.jpg|right|400px]] | ||
| + | #10 µl of '''BBa_K517003''' | ||
| + | #10 µl of '''BBa_K517003''' | ||
| + | #10 µl of '''BBa_K517003''' | ||
| + | #10 µl of '''BBa_K517003''' | ||
| + | #10 µl of '''BBa_K517003''' | ||
| + | #10 µl of '''p. cola''' | ||
| + | #10 µl of '''p. cola''' | ||
| + | #10 µl of '''p. cola''' | ||
| + | #10 µl of '''p. cola''' | ||
| + | #10 µl of '''p. cola''' | ||
| - | + | ====Purification of the previous PCR==== | |
| + | ''by Pierre'' | ||
| + | From the 5 tubes of p.Cola I cleaned up two tubes. | ||
| + | From the 5 tubes of BBa_K517003 I cleaned up two tubes. | ||
| - | + | I did an electroforesis to check if the clening has worked. It did. | |
| - | + | #1 µl of '''BBa_K517003''' | |
| + | #1 µl of '''p. cola''' | ||
| - | + | ====Stock of pcola plasmides==== | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | ==== | + | |
using the DNA kit extraction to extract pcola () | using the DNA kit extraction to extract pcola () | ||
| - | + | ==Human Practices== | |
| - | ==Human | + | |
===Art & Design=== | ===Art & Design=== | ||
''by Terry'' | ''by Terry'' | ||
| Line 47: | Line 77: | ||
The mold is cut delicately and the test piece removed. | The mold is cut delicately and the test piece removed. | ||
| - | [[File:Photo agar test 003.jpg| | + | [[File:Photo agar test 003.jpg|750px|center]] |
Then, the mold is placed back in the becher and filled up with regular agar ( 20mg/ml ) | Then, the mold is placed back in the becher and filled up with regular agar ( 20mg/ml ) | ||
| Line 58: | Line 88: | ||
Result : SUCCESS, I slightly remoistened the gel with water for an easier unmolding. The agar test piece is perfectly removed. | Result : SUCCESS, I slightly remoistened the gel with water for an easier unmolding. The agar test piece is perfectly removed. | ||
| - | [[File:Photo agar test 004.jpg| | + | [[File:Photo agar test 004.jpg|750px|center]] |
| + | |||
| + | ==Photo of the Day== | ||
| + | [[File:Paris Saclay 8_august.jpg|600px|center]] | ||
| + | |||
| + | |||
| + | '''Members present:''' | ||
| + | * Instructors and advisors: Alice, Solenne and Sylvie. | ||
| + | * Students: Fabio, Hoang Vu, Eugène, Juliette, Mélanie, Pierre Romain, Sean and Terry | ||
| + | |||
| + | {{Team:Paris_Saclay/notebook_footer}} | ||
Latest revision as of 14:49, 14 October 2014
Contents |
Friday 8th August
Lab work
Salicylate Inducible Suppressing System
Transformation of competent E.coli cells
by Fabio
We transformed in DH5α the Ligation's result made yesterday (BBa_K1372000 with BBa_B0015). The samples were put at 37°C for 10 hours, then we put them at room temperature until monday morning)
Samples:
- Sample 2 μl
- Sample 1 μl
- DH5α Control in LB
- DH5α Control in LB + Cm
Results on 11th August morning:
- Success, grown very well (> 200 colonies)
- Success, grown well (> 100 colonies)
- Success, a film of bacteria has settled
- Success, nothing has grown
Lemon Scent
PCR
by Mélanie & Pierre We have done a PCR with pcola (geranyl synthase) and BBa_K517003:
- Green GotaqBuffer = 10µl
- MgCl2 = 4µl
- dNTP = 1µl
- Primer A = 1µM
- Primer B = 1µM
- DNA = 1µl
- Gotaq DNA polymerase = 0.25µl
Oligo used : iPS81bis AND iPS82 (pcola) iPS69 and iPS68bis (BBa_K517003)
PCR cycles:
- 95° 2min
- 95° 30sec
- 49-55° 30 sec
- 72° 2min
- 71° 5min
Electrophoresis
- 10 µl of BBa_K517003
- 10 µl of BBa_K517003
- 10 µl of BBa_K517003
- 10 µl of BBa_K517003
- 10 µl of BBa_K517003
- 10 µl of p. cola
- 10 µl of p. cola
- 10 µl of p. cola
- 10 µl of p. cola
- 10 µl of p. cola
Purification of the previous PCR
by Pierre
From the 5 tubes of p.Cola I cleaned up two tubes. From the 5 tubes of BBa_K517003 I cleaned up two tubes.
I did an electroforesis to check if the clening has worked. It did.
- 1 µl of BBa_K517003
- 1 µl of p. cola
Stock of pcola plasmides
using the DNA kit extraction to extract pcola ()
Human Practices
Art & Design
by Terry
Sequel of yesterday molding attempt.
The mold is cut delicately and the test piece removed.
Then, the mold is placed back in the becher and filled up with regular agar ( 20mg/ml )
Result : FAILURE, the agar test piece has been ripped when unmolding. It was stuck to the mold.
But the mold is still intact ! I cleaned it up and slightly covered the inward surface with liquid soap. The mold is filled up again and conserved in the refrigerator for 2 hours.
Result : SUCCESS, I slightly remoistened the gel with water for an easier unmolding. The agar test piece is perfectly removed.
Photo of the Day
Members present:
- Instructors and advisors: Alice, Solenne and Sylvie.
- Students: Fabio, Hoang Vu, Eugène, Juliette, Mélanie, Pierre Romain, Sean and Terry
 "
"