Team:Colombia/Interlab
From 2014.igem.org
Camilog137 (Talk | contribs) |
Camilog137 (Talk | contribs) |
||
| (16 intermediate revisions not shown) | |||
| Line 38: | Line 38: | ||
<p> | <p> | ||
<br> | <br> | ||
| - | The fluorometric | + | The fluorometric measures were taken using a ISS PC1 Photon Counting Spectrofluorometer with magnetic stirring using the wavelength corresponding to GFP emission. The measures were carried out three times while 10 min with an ON culture of E. coli TOP 10 transformed with the device diluted in a 1:10 proportion in 2mL of fresh LB, with a previous measuring of OD. We used E. coli TOP 10 with no plasmids as blank. All measurement were taken at 37 °C. |
</p> | </p> | ||
| Line 52: | Line 52: | ||
<p> | <p> | ||
| - | + | Devices were comfirmed by profile digestion observed in agorose gel. The devices were digested with EcoRI and PstI. For allrestrictions we obtained two bands (the plasmid and the device) with the expected sizes in all cases. | |
</p> | </p> | ||
| Line 88: | Line 88: | ||
<html> | <html> | ||
</p> | </p> | ||
| + | <br><br> | ||
| + | You can find our worksheet <a href="https://2014.igem.org/File:Worksheet.pdf" target="_blank">here.</a> | ||
<br><br><br> | <br><br><br> | ||
| - | <b> <font size="10"> | + | <b> <font size="10"> Tested Parts </font> </b> |
<br><br> | <br><br> | ||
<b><font color="#8A0808" size="5" >BBa_I20260 (J23101-B0032-E0040-B0015)</font></b> | <b><font color="#8A0808" size="5" >BBa_I20260 (J23101-B0032-E0040-B0015)</font></b> | ||
| - | <br> | + | <br><br> |
<p> | <p> | ||
This part corresponds to GFP under the constitutive promoter J23101, with B0032 as RBS and two stops (B001 and B0010). The backbone of this already constructed device is pSB3K3 with kandamycin resistance. | This part corresponds to GFP under the constitutive promoter J23101, with B0032 as RBS and two stops (B001 and B0010). The backbone of this already constructed device is pSB3K3 with kandamycin resistance. | ||
| Line 105: | Line 107: | ||
</html> | </html> | ||
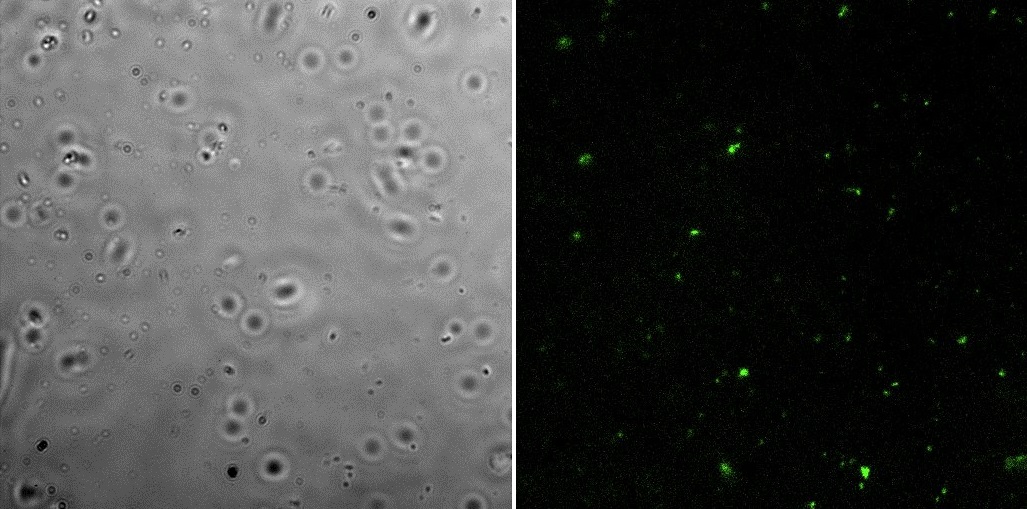
[[File:IL1comp.jpg|700px|thumb|center|Light (left) and FITC (right)]] | [[File:IL1comp.jpg|700px|thumb|center|Light (left) and FITC (right)]] | ||
| - | + | ||
<html> | <html> | ||
</center> | </center> | ||
| Line 119: | Line 121: | ||
</center> | </center> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | <br> | + | <br><br><br> |
<b><font color="#8A0808" size="5" >BBa_J23115 + BBa_E0240 (B0032-E0040-B0015)</font></b> | <b><font color="#8A0808" size="5" >BBa_J23115 + BBa_E0240 (B0032-E0040-B0015)</font></b> | ||
| - | <br> | + | <br><br> |
<p> | <p> | ||
This part is identical to the last one, but built by us on a pSB1C3 backbone. | This part is identical to the last one, but built by us on a pSB1C3 backbone. | ||
| Line 148: | Line 146: | ||
</center> | </center> | ||
| - | < | + | <br><br> |
| - | + | ||
| - | + | ||
| - | + | ||
<br> | <br> | ||
<b><font color="#8A0808" size="5" >BBa_J23101 + BBa_E0240 (B0032-E0040-B0015)</font></b> | <b><font color="#8A0808" size="5" >BBa_J23101 + BBa_E0240 (B0032-E0040-B0015)</font></b> | ||
| + | <br><br> | ||
<p> | <p> | ||
The only difference between this part and the last one is the constitutive promoter, which in this case is J23115. The plasmid, RBS and stops are all the same. | The only difference between this part and the last one is the constitutive promoter, which in this case is J23115. The plasmid, RBS and stops are all the same. | ||
| Line 177: | Line 173: | ||
</center> | </center> | ||
| - | <b> | + | |
| + | |||
| + | <br><br><br> | ||
| + | <b><font size="10"> Validation </font></b> | ||
| + | <br><br> | ||
| + | |||
<p> | <p> | ||
| - | + | Unfortunately, we got unexpected troubles with the spectrofluorometer for final measurements, so we only present in this page these measurements for one set of samples (first device). | |
| - | </ | + | <center> |
| + | </html> | ||
| + | [[File:sinod.jpg|700px|thumb|center|Measurements for each sample of BBa_I20260 (only intensity)]] | ||
| + | <html> | ||
| + | </center> | ||
| + | <br> | ||
| + | <center> | ||
| + | </html> | ||
| + | [[File:conod.jpg|700px|thumb|center|Measurements for each sample of BBa_I20260 (intensity/OD)]] | ||
| + | <html> | ||
| + | </center> | ||
| - | < | + | </p> |
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
| + | </div> | ||
| + | <center> | ||
| + | <div class="button-fill orange" ><div class="button-text">Back to Wet Lab</div><div class="button-inside"><div class="inside-text"><a style="text-decoration: none; background-color: none; color: red;" href="https://2014.igem.org/Team:Colombia/WetLab">Go! </a></div></div></div> | ||
| + | </center> | ||
| + | <br><br><br><br><br><br> | ||
</div> | </div> | ||
| + | <script> | ||
| + | $(".button-fill").hover(function () { | ||
| + | $(this).children(".button-inside").addClass('full'); | ||
| + | }, function() { | ||
| + | $(this).children(".button-inside").removeClass('full'); | ||
| + | }); | ||
| + | //@ sourceURL=pen.js | ||
| + | </script> | ||
</html> | </html> | ||
Latest revision as of 04:35, 11 October 2014
Interlab Study
This year Colombia Team-iGEM is participating in Interlab Study! We are glad of share our results and measurements. You can find them in the tabs below, you can find the specifications of our equipments and the methodology we used as well.
Methods
Strains and culture
All transformations were carried out in ''E. coli'' TOP10. This bacterium was cultured in LB (supplemented with the appropriate antibiotic for each case) at 37 °C.
Transformations and plasmid DNA extraction
This process was carried out as indicated in our protocols page.
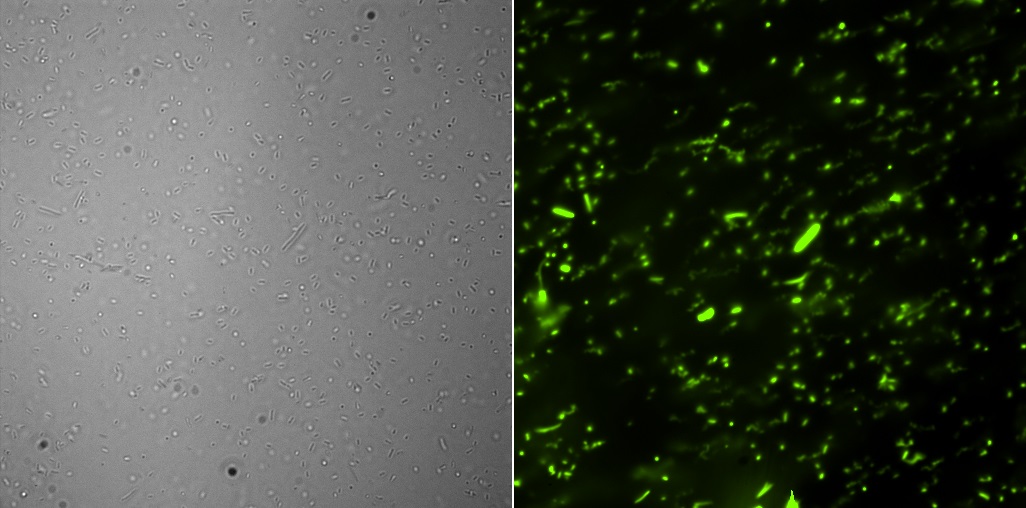
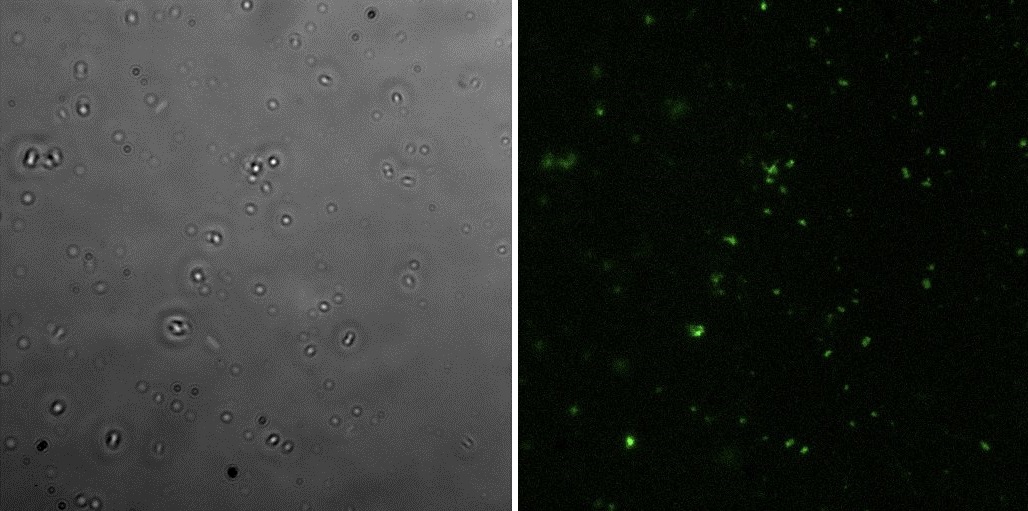
All the pictures shown here were taken using a Eclipse-Ti Nikon® ECLIPSE Ti inverted microscope equiped with an ANDOR iXon Ultra 897 camera. The filter used was FITC.
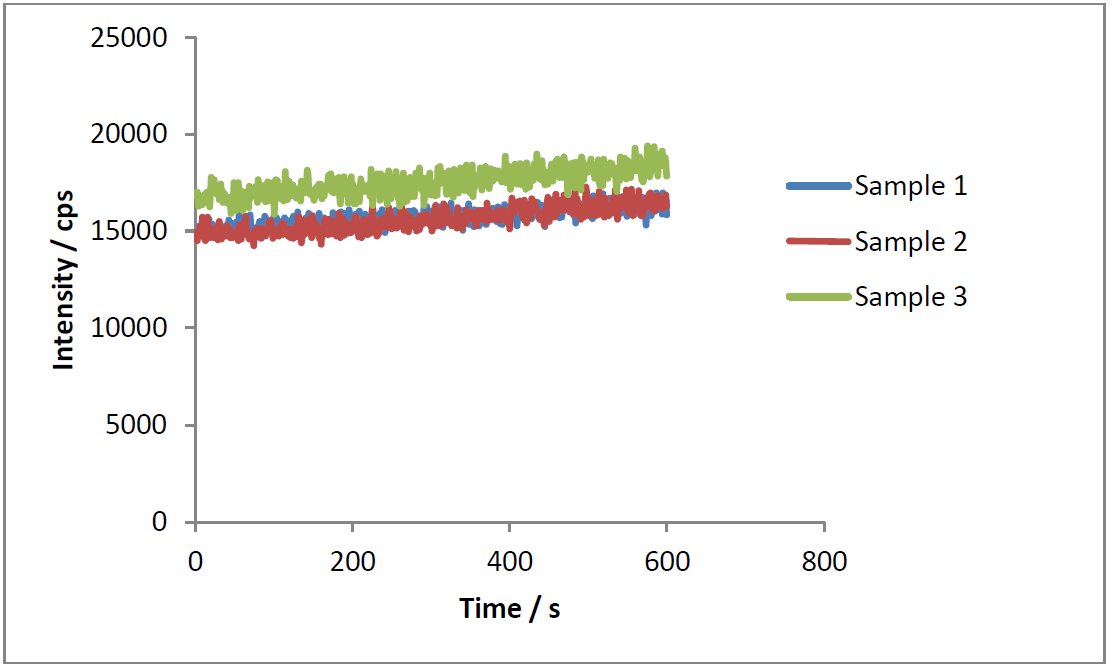
The fluorometric measures were taken using a ISS PC1 Photon Counting Spectrofluorometer with magnetic stirring using the wavelength corresponding to GFP emission. The measures were carried out three times while 10 min with an ON culture of E. coli TOP 10 transformed with the device diluted in a 1:10 proportion in 2mL of fresh LB, with a previous measuring of OD. We used E. coli TOP 10 with no plasmids as blank. All measurement were taken at 37 °C.
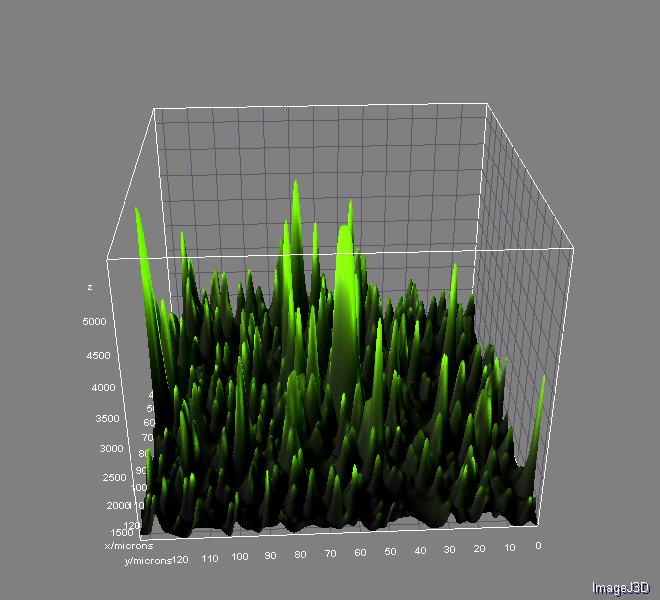
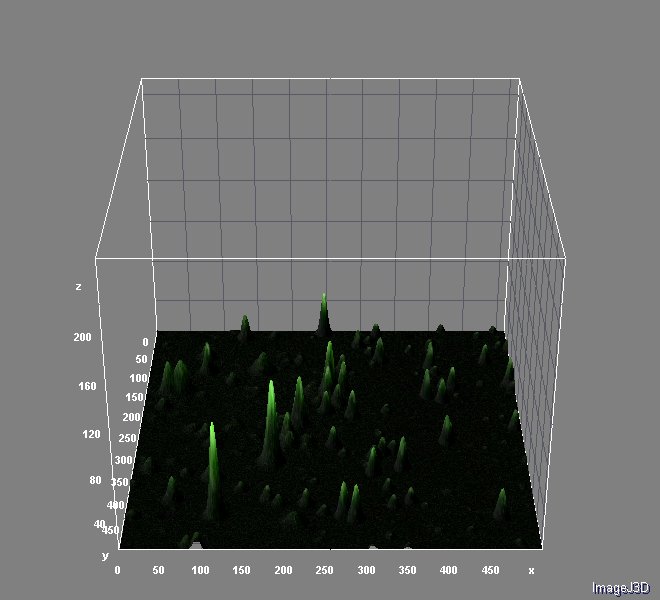
Image analyses were performed with ImageJ 1.46r using Nikon ND Reader and interactive 3D Surface Plot pluggins. We used ''Analyzing fluorescence microscopy images with ImageJ'' (Queen's University Belfast 2014) as guide. You can download this useful material here.
Validation
Devices were comfirmed by profile digestion observed in agorose gel. The devices were digested with EcoRI and PstI. For allrestrictions we obtained two bands (the plasmid and the device) with the expected sizes in all cases.
You can check the size of each device with the next table:
| Name | Backbone + device / bp | Device / bp | Linearized backbone / bp |
| BBa_I20260 | 3669 | 919 | 2750 |
| BBa_J23101 + BBa_E0240(B0032-E0040-B0015) | 2981 | 911 | 2070 |
| BBa_J23115 + BBa_E0240 (B0032-E0040-B0015) | 2981 | 911 | 2070 |
You can find our worksheet here.
Tested Parts
BBa_I20260 (J23101-B0032-E0040-B0015)
This part corresponds to GFP under the constitutive promoter J23101, with B0032 as RBS and two stops (B001 and B0010). The backbone of this already constructed device is pSB3K3 with kandamycin resistance.
Fluorescence microscopyHere are the images of the transformed cells with this device:
And the analysis for relative fluorescence among them performed from the last image above:
BBa_J23115 + BBa_E0240 (B0032-E0040-B0015)
This part is identical to the last one, but built by us on a pSB1C3 backbone.
Fluorescence microscopyHere are the images of the transformed cells with this device:
And the analysis for relative fluorescence among them performed from the last image above:
BBa_J23101 + BBa_E0240 (B0032-E0040-B0015)
The only difference between this part and the last one is the constitutive promoter, which in this case is J23115. The plasmid, RBS and stops are all the same.
Fluorescence microscopyHere are the images of the transformed cells with this device:
And the analysis for relative fluorescence among them performed from the last image above:
Validation
Unfortunately, we got unexpected troubles with the spectrofluorometer for final measurements, so we only present in this page these measurements for one set of samples (first device).
 "
"