Team:Paris Saclay/Notebook/August/22
From 2014.igem.org
(Difference between revisions)
(→pPSI dephosphorylation) |
(→pPSI dephosphorylation) |
||
| Line 31: | Line 31: | ||
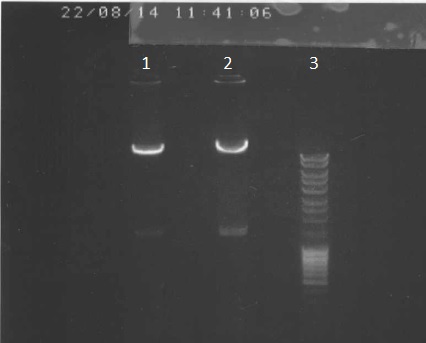
We've made a electrophoresis to check the digestion. | We've made a electrophoresis to check the digestion. | ||
| + | |||
| + | |||
| + | '''Legend''' | ||
| + | #pPSI+Apra digested via PacI, dephosphorylated 5µl | ||
| + | #pPSI+Apra digested via PacI, dephosphorylated 5µl | ||
| + | #ladder 5µl | ||
[[File:Paris_Saclay_140822.jpg]] | [[File:Paris_Saclay_140822.jpg]] | ||
Revision as of 10:05, 25 August 2014
Contents |
Friday 22nd August
Lab work
D - Lemon Scent
pPSI digestion
| components | volumes |
|---|---|
| pPSI | 40μl |
| FastDigest green buffer 10X | 5μl |
| PacI | 2µl |
| H2O | 2µl |
pPSI dephosphorylation
After 2 hours, add 1.5µL of Alkaline Phosphatase (AP) and let it 1 hour.
Then, inactivation of AP at 65°C during 15 minutes.
We've made a electrophoresis to check the digestion.
Legend
- pPSI+Apra digested via PacI, dephosphorylated 5µl
- pPSI+Apra digested via PacI, dephosphorylated 5µl
- ladder 5µl
Ligation of BBa_K517003, BBa_K762100, and p cola in pPSI
| components | volumes |
|---|---|
| BBa_K517003 | 10μl |
| pPSI | 2μl |
| buffer | 2µl |
| H2O | 5µl |
| ligase | 1µl |
| components | volumes |
|---|---|
| BBa_K762100 | 10μl |
| pPSI | 2μl |
| buffer | 2µl |
| H2O | 5µl |
| ligase | 1µl |
| components | volumes |
|---|---|
| p cola | 10μl |
| pPSI | 2μl |
| buffer | 2µl |
| H2O | 5µl |
| ligase | 1µl |
And let it in the freezer for 3 hours.
Transformation in competent E.coli DH5α
After 3 hours, take out the ligation tubes and add 100 µl of competent DH5α in each tube.
Then proceed to the transformation protocol.
- 20 minutes at 4°C
- 2 minutes 30 seconds at 42°C
- 2 minutes at 4 °C
- Add 0.9 ml of LB into the tubes
Finally put it in a incubator at 37°C for 30 minutes
 "
"