Team:Paris Saclay/Modeling/Fusion Proteine
From 2014.igem.org

Contents |
Fusion Protein
Countdown
This page is under Arnaud's responsibility.
- Deadline: 08/oct.
- Final text
- Deadline: 12/oct.
- Final review.
Overview
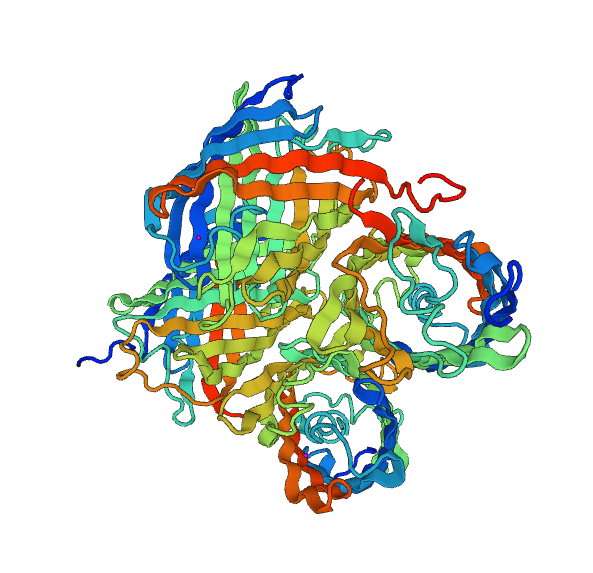
Here we will discuss the structural modeling of the fusion protein that we had planned in order to obtain a green chromoprotein. We used the bioinformatics tools developed by the Swiss Institute of Bioinformatics (Swiss-Model [1]) and by the Xu group of Chicago University (RaptorX [2]).
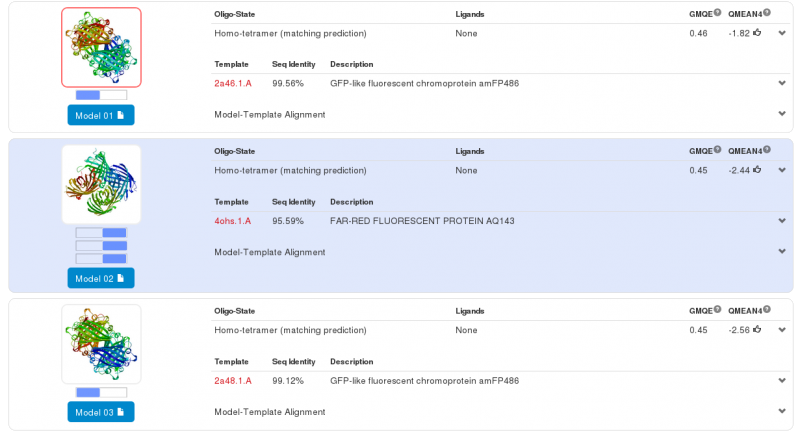
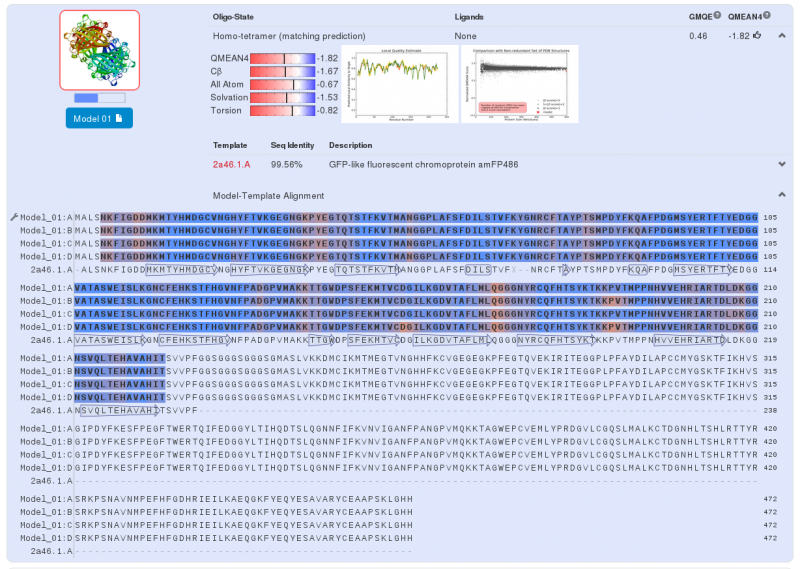
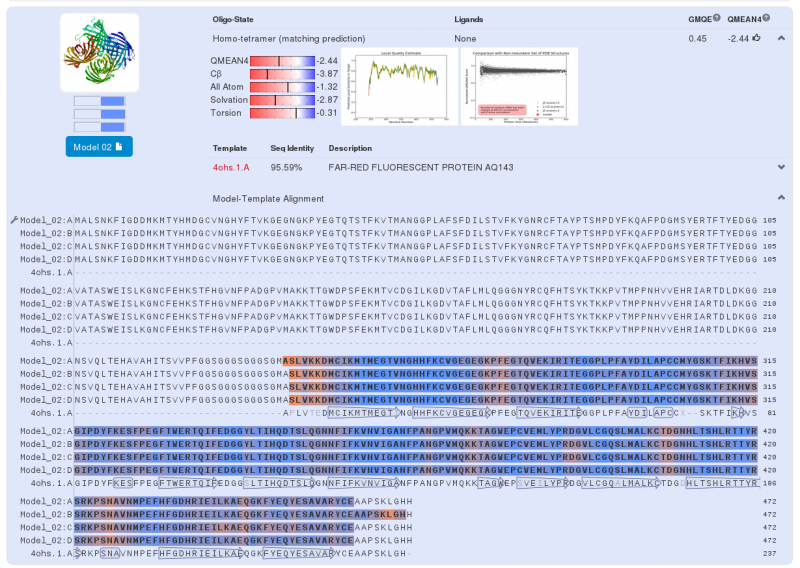
Swiss-Model has given us three highly probable models. Two models for the first part of our Fusion Protein and another for second part of our protein. We chose the first model for the yellow chromoprotein (1st part) and the second model for the blue chromoprotein (2nde part).
The first part – Yellow chromoprotein :
The yellow chromoprotein used to create this fusion protein is the amajLime a yellow-green chromoprotein (Bba_K1033916 develloped by iGEM13_Uppsala). Homology modeling via Swiss-Model for the first chromoprotein gives us a quasi exact match with amFP486 which is a cyan fluorescent protein from Anemonia majano (the same organism as our biobrick). Only 3 amino-acids change, these amino-acids are in the core of the protein. We can therefore hypothesize that at least three positions can be modified to change the emission spectrum of the chromoprotein.
Note that this chromoprotein is a homotetramer. What might be able to raise problems is if homotetramerie was essential to the functionality of the protein. The literature[3] tells us that it does not appear to be the case, since fusion proteins have already been created with chromoproteins, which were homo-multimeric and the protein fusions were completely functional.
We predict that in our project the homotetramerie is not important to the functionality of our fusion protein, it is believed that the light emission will be less.
The second part – blue chromoprotein :
We can do the same analysis for the blue chromoprotein. For the second part of the fusion protein we use to the aeBlue which is a blue chromoprotein (Bba_K1033929 develloped by iGEM13_Uppsala). Swiss-Model gives us a model for the second part; a near perfect match between our protein sequence and chromoprotein (far-red fluorescent protein AQ143). There is a 95.59% similarity between the two sequences, the major differences are present at the C-terminus and N-terminus. As for the Yellow chromoprotein, three amino acids of the core protein are not the same.
Note that this chromoprotein is a homotetramer.
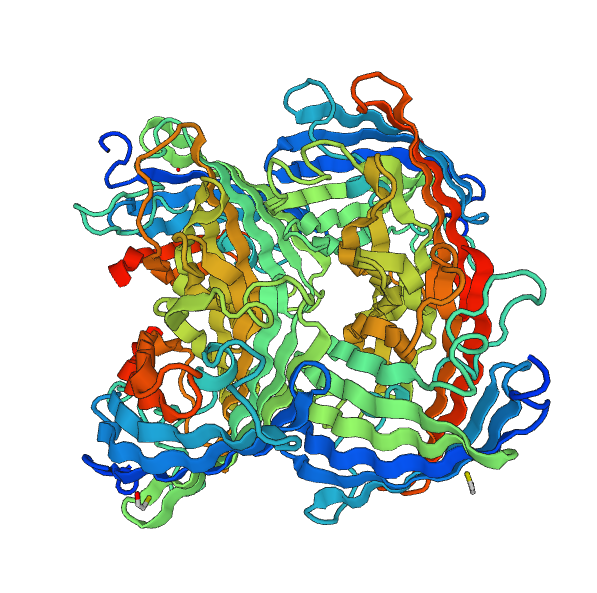
Modeling with Swiss-Model does not provide information on the overall structure of our chromoprotein. Nonetheless, it gives us a very good approach to the structure of two chromoproteins which make up our fusion protein.
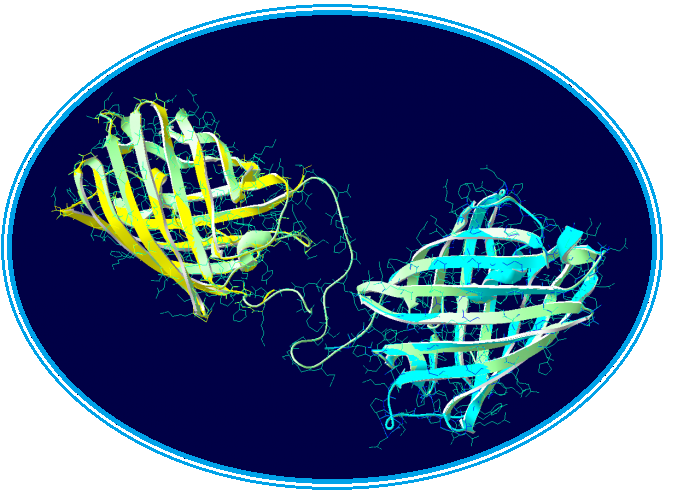
The last part – Global modelisation :
We could make a much more complex modelisation for the overall structure of the fusion protein, we think it is unnecessary. Therefore, to try to have a global vision of our protein we will use the RaptorX model.
Once this new model was obtained, we performed structural alignments and we discovered the structure of the two chromoproteins, so it seems that the linker between the two proteins is not sufficient to induce stress on the structures of our fusion protein.
Obviously many biases have been introduced during this modeling and the conception of the fusion protein. This modelisation is closer to a structural analysis that a complex protein modeling. The assumptions used in the design of this chromoprotein (fusion protein) does not seem to be so far from reality. We found similar cases in scientific publications, which are perfectly functional. We found several hypothesis that explain the failure of our experience. The main hypothesis is an error in the design of the nucleotide sequence that is requested to synthesize.
References
[1] [http://swissmodel.expasy.org/ Swiss-Model server]
[2] Protein Structure and Function Prediction, [http://raptorx.uchicago.edu/ RaptorX server], Chicago university
[3] [http://nar.oxfordjournals.org/content/33/5/e49.full.pdf+html A dual-fluorescence reporter system for high-throughput clone characterization and selection by cell sorting] - Juno Choe, Haiwei H. Guo and Ger van den Engh, Nucleic Acids Research, 2005, Vol. 33, No. 5
 "
"