Team:Paris Saclay/Modeling
From 2014.igem.org
(→Modeling) |
(→Modeling) |
||
| Line 3: | Line 3: | ||
=Modeling= | =Modeling= | ||
| + | |||
| + | |||
| + | [https://2014.igem.org/Team:Paris_Saclay/Modeling/test] | ||
To realise our artwork, we use an agarose gel to obtain the shape of a lemon. To push the resemblance to the extreme, we wish to have a crust in the edge of the lemon when we seperate it. In fact, we build bacteria who produce yellow/green color in presence of oxygen. Thus we must evaluate the penetration of the oxygen in the gel ! | To realise our artwork, we use an agarose gel to obtain the shape of a lemon. To push the resemblance to the extreme, we wish to have a crust in the edge of the lemon when we seperate it. In fact, we build bacteria who produce yellow/green color in presence of oxygen. Thus we must evaluate the penetration of the oxygen in the gel ! | ||
Revision as of 18:41, 30 September 2014
Modeling
To realise our artwork, we use an agarose gel to obtain the shape of a lemon. To push the resemblance to the extreme, we wish to have a crust in the edge of the lemon when we seperate it. In fact, we build bacteria who produce yellow/green color in presence of oxygen. Thus we must evaluate the penetration of the oxygen in the gel !
Oxygen penetrates into the gel by diffusion phenomenon that we will study below. We first use the following phenomenological law suggest by Adolphe Fick in 1855:
In an homogeneous and isotropic environment, containing particles distributed inhomogeneously,appears spontaneously a volumetric flow density vector particle $\overrightarrow{J}(M,t) $. In any point $M$ in space, this vector is proportional to the gradient of the particle density $n(M,t)$. Mathematicaly, this relationship take the form: \[ \overrightarrow{J}(M,t) = - D \times \nabla n(M,t) \qquad (1) \] where $D$ is the diffusion coefficient and $\nabla$ is the gradient vector.
In equation after Fick's law above, we need to know the parameter $D$. We then searched in scientific literature and article [1] describes a method to get it. Referring to this article, the diffusion coefficient of oxygen in agarose is $ D = 0{,}256 \times 10^{-8} m^2 s^{-1} $.
The phenomenon we are facing is quite difficult to tackle if taken as a whole and We must emit a number of intuitively acceptable hypothesis.
- To simplify the problem, we consider that the diffusion of oxygen particle occurs only in one direction. So $\overrightarrow{J}(M,t) = J(x,t) \overrightarrow{e}_x $. This hypothesis is credible because we seek the maximum penetrance of oxygen. This is why we consider that the diffusion occurs in the line of greatest slope.
- Spatial variations in the density of particles are connected to spatial variations of the vector $\overrightarrow{J}(M,t)$ by the material's equation of conservation in presence of volume distribution of particle source $\sigma (x,t)$ (device which injects or subtracted particles to the system) :
\[ \frac{\partial n}{\partial t} (x,t) = - \frac{\partial J}{\partial x} (x,t) + \sigma (x,t) \qquad (2) \]
By replacing $(2)$ in $(1)$, we obtain the following equation of diffusion : \[ \forall t, \forall x, \bigg( \frac{\partial}{\partial t} - D \frac{\partial^2}{\partial x^2} \bigg) n(x,t) = \sigma (x,t) \qquad (3) .\]
As our lemon is exposed to the ambient air, we stay in steady state where the source $ \sigma (x,t) $ is equal to $N_0$ the quantity of $O_2$ in the air.
To solve this equation, we use Fourier's analysis and it is kwown (classical solution of the heat equation) that
\[ \forall x, \forall t>0, n(x,t) = \frac{N_0}{\sqrt{4 \pi D t}} exp \bigg(- \frac{x^2}{4 D t} \bigg) + \int_{0}^{t} \underbrace{N_0 * exp \bigg(- \frac{|x|^2}{4 D \tau} \bigg)}_{= 0 \text{ by symmetry of the gaussian distribution }} \frac{d\tau}{\sqrt{4 \pi D \tau} } \]
Whence, \[ \forall x, \forall t>0, n(x,t) = \frac{N_0}{\sqrt{4 \pi D t}} exp \bigg(- \frac{x^2}{4 D t} \bigg) \]
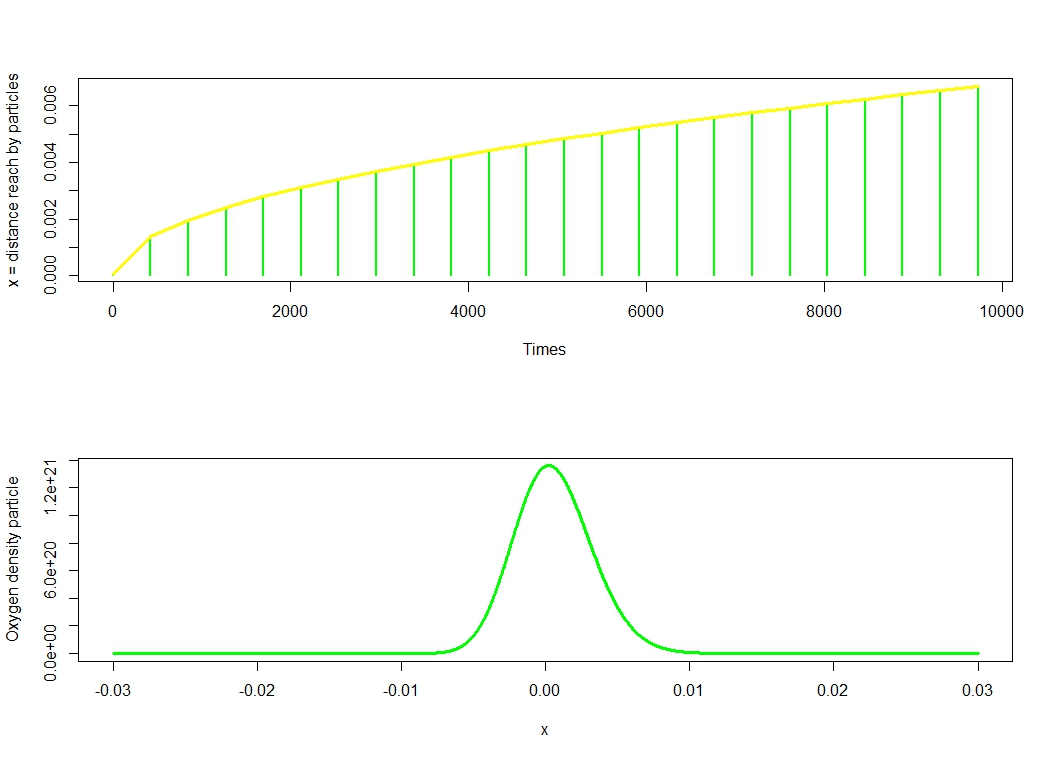
Thus we deduce easily that the average dispersion particle is given by the variance $\Delta x = \sqrt{2Dt}$. Using this formula, we deduct that, for example, oxygen will penetrate $3 \times 10^{-3} m$ in $1956.522 s = 32.6082 $ minutes.
The charts below give us a very clear idea of penetrance evaluated over time.
Graphical visualization
[1] A.C. Hulst, H.J.H. Hens, R.M. Buitelaar and J. Tramper, Determination of the effective diffusion coefficient of oxygen in gel materials in relation to gel concentration, Biotechnology Techniques Vol 3 No 3 199-204 (1989).
[2] Vincent Renvoizé, Physique PC-PC*, Cap Prepas, Pearson Education, 2010.
 "
"