Team:KIT-Kyoto/Notebook/Labnote
From 2014.igem.org
| (18 intermediate revisions not shown) | |||
| Line 24: | Line 24: | ||
<li class="category"> | <li class="category"> | ||
<a href="javascript:void(0)"> | <a href="javascript:void(0)"> | ||
| - | <font color="#143"> | + | <font color="#143">Click Here To Enlarge</font> LabNote |
</a> | </a> | ||
</li> | </li> | ||
| Line 220: | Line 220: | ||
<td>pGEX6P-2</td> | <td>pGEX6P-2</td> | ||
<td>CuMTSE1</td> | <td>CuMTSE1</td> | ||
| - | <td> | + | <td>Non Treated</td> |
</tr> | </tr> | ||
<tr> | <tr> | ||
| Line 241: | Line 241: | ||
<td>pGEX6P-2</td> | <td>pGEX6P-2</td> | ||
<td>CuMTSE2</td> | <td>CuMTSE2</td> | ||
| - | <td> | + | <td>Non Treated</td> |
</tr> | </tr> | ||
<tr> | <tr> | ||
| Line 262: | Line 262: | ||
<td>pGEX6P-2</td> | <td>pGEX6P-2</td> | ||
<td>CuMTS3</td> | <td>CuMTS3</td> | ||
| - | <td> | + | <td>Non Treated</td> |
</tr> | </tr> | ||
<tr> | <tr> | ||
| Line 283: | Line 283: | ||
<td>pGEX6P-2</td> | <td>pGEX6P-2</td> | ||
<td>CuMTS62</td> | <td>CuMTS62</td> | ||
| - | <td> | + | <td>Non Treated</td> |
</tr> | </tr> | ||
<tr> | <tr> | ||
| Line 755: | Line 755: | ||
<!--AGE--> | <!--AGE--> | ||
<br> | <br> | ||
| - | + | Apply λDNA-HindⅢ to compare and contrast AGE of July 14 and June 11. | |
<br> | <br> | ||
<img src="/wiki/images/b/b6/Kit_June14_c.jpg"> | <img src="/wiki/images/b/b6/Kit_June14_c.jpg"> | ||
| Line 1,299: | Line 1,299: | ||
<!-- 実験者名終わり --> | <!-- 実験者名終わり --> | ||
<br> | <br> | ||
| - | + | Cultivate following samples in Big Scale at 30℃ overnight in shaken culture. | |
<br> | <br> | ||
<div class="m_table"> | <div class="m_table"> | ||
| Line 1,331: | Line 1,331: | ||
<br> | <br> | ||
| - | <!--DNA | + | <!--DNA Refinement 1--> |
| - | <strong>DNA | + | <strong>DNA Refinement 1</strong> |
<div class="m_table"> | <div class="m_table"> | ||
<table class="materials"> | <table class="materials"> | ||
| Line 1,351: | Line 1,351: | ||
</table> | </table> | ||
</div> | </div> | ||
| - | <!--DNA | + | <!--DNA Refinement 1終わり--> |
<br> | <br> | ||
| Line 1,396: | Line 1,396: | ||
<br> | <br> | ||
| - | <!--DNA | + | <!--DNA Refinement 2--> |
| - | <strong>DNA | + | <strong>DNA Refinement 2</strong> |
<div class="m_table"> | <div class="m_table"> | ||
<table class="materials"> | <table class="materials"> | ||
| Line 1,429: | Line 1,429: | ||
</table> | </table> | ||
</div> | </div> | ||
| - | <!--DNA | + | <!--DNA Refinement 2--> |
<br> | <br> | ||
| Line 1,984: | Line 1,984: | ||
<td></td> | <td></td> | ||
<td></td> | <td></td> | ||
| - | <td> | + | <td>Non Treated</td> |
</tr> | </tr> | ||
<tr> | <tr> | ||
| Line 2,016: | Line 2,016: | ||
<td></td> | <td></td> | ||
<td></td> | <td></td> | ||
| - | <td> | + | <td>Non Treated</td> |
</tr> | </tr> | ||
<tr> | <tr> | ||
| Line 2,040: | Line 2,040: | ||
<td></td> | <td></td> | ||
<td></td> | <td></td> | ||
| - | <td> | + | <td>Non Treated</td> |
</tr> | </tr> | ||
</table> | </table> | ||
| Line 2,071: | Line 2,071: | ||
<td>p427-TEF</td> | <td>p427-TEF</td> | ||
<td>CuMTSE1</td> | <td>CuMTSE1</td> | ||
| - | <td>pGEX6P-2F2, pGEX6P-2R<td> | + | <td>pGEX6P-2F2, pGEX6P-2R</td> |
</tr> | </tr> | ||
</table> | </table> | ||
| Line 2,313: | Line 2,313: | ||
<td></td> | <td></td> | ||
<td></td> | <td></td> | ||
| - | <td> | + | <td>Non Treated</td> |
</tr> | </tr> | ||
<tr> | <tr> | ||
| Line 2,321: | Line 2,321: | ||
<td>CuMTSE1</td> | <td>CuMTSE1</td> | ||
<td>pGEX6P-2F1, pGEX6P-2R</td> | <td>pGEX6P-2F1, pGEX6P-2R</td> | ||
| - | <td> | + | <td>Non Treated</td> |
</tr> | </tr> | ||
<tr> | <tr> | ||
| Line 2,329: | Line 2,329: | ||
<td>CuMTSE1</td> | <td>CuMTSE1</td> | ||
<td>pGEX6P-2F2, pGEX6P-2R</td> | <td>pGEX6P-2F2, pGEX6P-2R</td> | ||
| - | <td> | + | <td>Non Treated</td> |
</tr> | </tr> | ||
<tr> | <tr> | ||
| Line 3,027: | Line 3,027: | ||
<td>六</td> | <td>六</td> | ||
<td>p427-TEF</td> | <td>p427-TEF</td> | ||
| - | <td> | + | <td>CuMTSE1</td> |
<td>pGEX6P-2F2, pGEX6P-2R</td> | <td>pGEX6P-2F2, pGEX6P-2R</td> | ||
</tr> | </tr> | ||
| Line 3,047: | Line 3,047: | ||
SOGA<br> | SOGA<br> | ||
<!-- 実験者名終わり --> | <!-- 実験者名終わり --> | ||
| - | <!--DNA | + | <!--DNA Refinement 1--> |
| - | <strong>DNA | + | <strong>DNA Refinement 1</strong> |
<br> | <br> | ||
<div class="m_table"> | <div class="m_table"> | ||
| Line 3,076: | Line 3,076: | ||
</table> | </table> | ||
</div> | </div> | ||
| - | <!--DNA | + | <!--DNA Refinement 1--> |
<br> | <br> | ||
| Line 3,133: | Line 3,133: | ||
<br> | <br> | ||
| - | <!--DNA | + | <!--DNA Refinement 2--> |
| - | <strong>DNA | + | <strong>DNA Refinement 2</strong> |
<div class="m_table"> | <div class="m_table"> | ||
<table class="materials"> | <table class="materials"> | ||
| Line 3,157: | Line 3,157: | ||
</table> | </table> | ||
</div> | </div> | ||
| - | <!--DNA | + | <!--DNA Refinement 2--> |
<br> | <br> | ||
| Line 3,191: | Line 3,191: | ||
DNA ligase: DNA Ligation Kit ‹Mighty Mix› (TAKARA BIO INC.) | DNA ligase: DNA Ligation Kit ‹Mighty Mix› (TAKARA BIO INC.) | ||
<br> | <br> | ||
| - | <!--DNA | + | <!--DNA Refinement 2--> |
<br> | <br> | ||
| Line 3,750: | Line 3,750: | ||
<td>CuMTS3</td> | <td>CuMTS3</td> | ||
<td>pGEX6P-2F1, pGEX6P-2R</td> | <td>pGEX6P-2F1, pGEX6P-2R</td> | ||
| - | <td> | + | <td>Non Treated</td> |
</tr> | </tr> | ||
<tr> | <tr> | ||
| Line 3,764: | Line 3,764: | ||
<td>CuMTS3</td> | <td>CuMTS3</td> | ||
<td>pGEX6P-2F1, pGEX6P-2R</td> | <td>pGEX6P-2F1, pGEX6P-2R</td> | ||
| - | <td> | + | <td>Non Treated</td> |
</tr> | </tr> | ||
<tr> | <tr> | ||
| Line 3,778: | Line 3,778: | ||
<td>CuMTS3</td> | <td>CuMTS3</td> | ||
<td>pGEX6P-2F1, pGEX6P-2R</td> | <td>pGEX6P-2F1, pGEX6P-2R</td> | ||
| - | <td> | + | <td>Non Treated</td> |
</tr> | </tr> | ||
<tr> | <tr> | ||
| Line 3,792: | Line 3,792: | ||
<td>CuMTS62</td> | <td>CuMTS62</td> | ||
<td>pGEX6P-2F1, pGEX6P-2R</td> | <td>pGEX6P-2F1, pGEX6P-2R</td> | ||
| - | <td> | + | <td>Non Treated</td> |
</tr> | </tr> | ||
<tr> | <tr> | ||
| Line 3,806: | Line 3,806: | ||
<td>CuMTS62</td> | <td>CuMTS62</td> | ||
<td>pGEX6P-2F1, pGEX6P-2R</td> | <td>pGEX6P-2F1, pGEX6P-2R</td> | ||
| - | <td> | + | <td>Non Treated</td> |
</tr> | </tr> | ||
<tr> | <tr> | ||
| Line 3,820: | Line 3,820: | ||
<td>CuMTS62</td> | <td>CuMTS62</td> | ||
<td>pGEX6P-2F1, pGEX6P-2R</td> | <td>pGEX6P-2F1, pGEX6P-2R</td> | ||
| - | <td> | + | <td>Non Treated</td> |
</tr> | </tr> | ||
<tr> | <tr> | ||
| Line 3,848: | Line 3,848: | ||
KOBAYASHI | KOBAYASHI | ||
<br> | <br> | ||
| - | + | Conduct mutagenesis to remove an illegal restriction site (<em>Eco</em>R1) | |
<br> | <br> | ||
<strong>InversePCR</strong> | <strong>InversePCR</strong> | ||
| Line 3,871: | Line 3,871: | ||
</div> | </div> | ||
(TOYOBO, KOD-Plus-Mutagenesis Kit) | (TOYOBO, KOD-Plus-Mutagenesis Kit) | ||
| - | + | Start from KOD -Plus- Mutagenesis Kit protocol No.2. | |
<br> | <br> | ||
<br> | <br> | ||
| Line 3,881: | Line 3,881: | ||
KOBAYASHI | KOBAYASHI | ||
<br> | <br> | ||
| - | + | Restart mutagenesis from KOD -Plus- Mutagenesis Kit protocol No.3 to obtain plasmid (muta-CuMTS3 in pGEX-6P-2) | |
<br> | <br> | ||
<strong>Transformation</strong> | <strong>Transformation</strong> | ||
| Line 3,912: | Line 3,912: | ||
<br> | <br> | ||
<strong>Colony Isolation</strong><br> | <strong>Colony Isolation</strong><br> | ||
| - | + | Isolate each colony from two plates which were inoculated overnight | |
<div class="m_table"> | <div class="m_table"> | ||
<table class="materials"> | <table class="materials"> | ||
| Line 3,934: | Line 3,934: | ||
</table> | </table> | ||
</div> | </div> | ||
| - | Select 5 isolated colonies whose code names are あ,か,さ,た and な and | + | Select 5 isolated colonies whose code names are あ,か,さ,た and な and miniprep them. |
<br> | <br> | ||
<br> | <br> | ||
| Line 4,115: | Line 4,115: | ||
</table> | </table> | ||
</div> | </div> | ||
| + | <img src="/wiki/images/b/bc/Kit_August22_c.jpg"> | ||
An illegal <em>Eco</em>R1 restriction site was removed.<br> | An illegal <em>Eco</em>R1 restriction site was removed.<br> | ||
<br> | <br> | ||
| Line 4,133: | Line 4,134: | ||
<td>muta-CuMTS3</td> | <td>muta-CuMTS3</td> | ||
<td>terpinene3-prefix and terpinene3-suffix</td> | <td>terpinene3-prefix and terpinene3-suffix</td> | ||
| - | <td>KOD-FX- | + | <td>KOD-FX-NEO-Plus</td> |
</tr> | </tr> | ||
</table> | </table> | ||
| Line 4,166: | Line 4,167: | ||
<td>muta-CuMTS3</td> | <td>muta-CuMTS3</td> | ||
<td>terpinene3-prefix and terpinene3-suffix</td> | <td>terpinene3-prefix and terpinene3-suffix</td> | ||
| - | <td>KOD-FX- | + | <td>KOD-FX-NEO-Plus</td> |
</tr> | </tr> | ||
</table> | </table> | ||
</div> | </div> | ||
| + | <img src="/wiki/images/7/72/Kit_August22_1_c.jpg"> | ||
<br> | <br> | ||
<br> | <br> | ||
| Line 4,228: | Line 4,230: | ||
</div> | </div> | ||
<br> | <br> | ||
| + | <img src="/wiki/images/3/31/Kit_August30_c.jpg"> | ||
Made total amount of 295 μL of PCR samples. | Made total amount of 295 μL of PCR samples. | ||
<br> | <br> | ||
| Line 4,233: | Line 4,236: | ||
<br> | <br> | ||
</p> | </p> | ||
| + | </div> | ||
| + | </div> | ||
<div class="accordion"> | <div class="accordion"> | ||
| Line 4,353: | Line 4,358: | ||
</table> | </table> | ||
</div> | </div> | ||
| + | <img src="/wiki/images/2/29/Kit_September18_c.jpg"> | ||
<br> | <br> | ||
<strong>PCR</strong> | <strong>PCR</strong> | ||
| Line 4,648: | Line 4,654: | ||
</table> | </table> | ||
</div> | </div> | ||
| + | <img src="/wiki/images/4/40/Kit_September19_c.jpg"> | ||
<br> | <br> | ||
Targeted colonies were identified in セ and ソ. | Targeted colonies were identified in セ and ソ. | ||
| Line 4,770: | Line 4,777: | ||
<br> | <br> | ||
</tr> | </tr> | ||
| + | |||
| + | <img src="/wiki/images/e/e6/Kit_September19_1_c.jpg"> | ||
<br> | <br> | ||
Targeted colonies were identified in colony N and F. | Targeted colonies were identified in colony N and F. | ||
<br> | <br> | ||
<br> | <br> | ||
| - | + | Select セ, ソ, N, F | |
<br> | <br> | ||
<br> | <br> | ||
| Line 4,817: | Line 4,826: | ||
</div> | </div> | ||
<br> | <br> | ||
| - | + | Cultivate these samples in 3mL of LB medium with ChlPhe at 30°C overnight. | |
<br> | <br> | ||
</p> | </p> | ||
| Line 5,153: | Line 5,162: | ||
</div> | </div> | ||
<br> | <br> | ||
| - | </ | + | <img src="/wiki/images/e/e5/Kit_September20_c.jpg"> |
| + | <div class="clear"><hr /></div> | ||
Sample F was selected as a submission plasmid. | Sample F was selected as a submission plasmid. | ||
<br> | <br> | ||
| Line 5,191: | Line 5,201: | ||
</div> | </div> | ||
<br> | <br> | ||
| - | + | Cultivate these samples in 3mL of LB medium with ChlPhe at 30°C overnight. | |
<br> | <br> | ||
</p> | </p> | ||
| Line 5,556: | Line 5,566: | ||
</tr> | </tr> | ||
</table> | </table> | ||
| - | </div | + | </div> |
| - | </ | + | <img src="/wiki/images/d/d7/Kit_September21_c.jpg"> |
<br> | <br> | ||
| Line 5,693: | Line 5,703: | ||
</div> | </div> | ||
<br> | <br> | ||
| - | + | Cultivate these samples in 3mL of SD medium with G418 at 30°C overnight. | |
<br> | <br> | ||
<br> | <br> | ||
| Line 5,740: | Line 5,750: | ||
</div> | </div> | ||
<br> | <br> | ||
| - | </ | + | <img src="/wiki/images/e/e0/Kit_October2_c.jpg"> |
</p> | </p> | ||
| Line 5,748: | Line 5,758: | ||
<strong>Main culture</strong> | <strong>Main culture</strong> | ||
<br> | <br> | ||
| - | + | Cultivate following samples in Big Scale with G418 at 28°C overnight in shaken culture. | |
<br> | <br> | ||
<div class="m_table"> | <div class="m_table"> | ||
| Line 6,059: | Line 6,069: | ||
</div> | </div> | ||
<br> | <br> | ||
| - | + | Cultivate samples in 3mL of SD medium with G418 at 30°C overnight. | |
<br> | <br> | ||
</tr> | </tr> | ||
| Line 6,197: | Line 6,207: | ||
KOBAYASHI | KOBAYASHI | ||
<br> | <br> | ||
| - | <strong>Main | + | <strong>Main Culture</strong> |
<br> | <br> | ||
<div class="m_table"> | <div class="m_table"> | ||
| Line 6,232: | Line 6,242: | ||
KOBAYASHI | KOBAYASHI | ||
<br> | <br> | ||
| - | <strong>GC | + | <strong>GC Analysis</strong> |
<br> | <br> | ||
<div class="m_table"> | <div class="m_table"> | ||
| Line 6,260: | Line 6,270: | ||
</div> | </div> | ||
<br> | <br> | ||
| - | </ | + | <img src="/wiki/images/b/b1/Kit_October10_c.jpg"> |
| - | </ | + | <div class="clear"><hr /></div> |
| + | For More Information: <a href="http://parts.igem.org/wiki/index.php?title=Part:BBa_K1448000">BBa_K1448000</a> | ||
</p> | </p> | ||
</div> | </div> | ||
Latest revision as of 01:13, 18 October 2014


LabNote
June
June 10, 2014
OKAMOTO
Miniprep
| Host | Sample | |
|---|---|---|
| Vector | Insert | |
| BL21(DE3) | pGEX6P-2 | CuMTSE1 |
| BL21(DE3) | pGEX6P-2 | CuMTSE2 |
| BL21(DE3) | pGEX6P-2 | CuMTS3 |
| BL21(DE3) | pGEX6P-2 | CuMTS62 |
Restriction Digest
| Host | Sample | Enzyme | Buffer | |
|---|---|---|---|---|
| Vector | Insert | |||
| BL21(DE3) | pGEX6P-2 | CuMTSE1 | EcoR1 | Buffer H |
| BL21(DE3) | pGEX6P-2 | CuMTSE2 | EcoR1 | Buffer H |
| BL21(DE3) | pGEX6P-2 | CuMTS3 | EcoR1 | Buffer H |
| BL21(DE3) | pGEX6P-2 | CuMTS62 | EcoR1 | Buffer H |
| BL21(DE3) | pGEX6P-2 | CuMTSE1 | Sal1, Not1 | Buffer H |
| BL21(DE3) | pGEX6P-2 | CuMTSE2 | Sal1, Not1 | Buffer H |
| BL21(DE3) | pGEX6P-2 | CuMTS3 | Sal1, Not1 | Buffer H |
| BL21(DE3) | pGEX6P-2 | CuMTS62 | Sal1, Not1 | Buffer H |
June 11, 2014
KOBAYASHI
AGE
| Lane | Sample | Enzyme | ||
|---|---|---|---|---|
| Marker | Vector | Insert | ||
| 1 | λDNA-HindⅢ | |||
| 2 | pGEX6P-2 | CuMTSE1 | Non Treated | |
| 3 | pGEX6P-2 | CuMTSE1 | EcoR1 | |
| 4 | pGEX6P-2 | CuMTSE1 | Sal1, Not1 | |
| 5 | pGEX6P-2 | CuMTSE2 | Non Treated | |
| 6 | pGEX6P-2 | CuMTSE2 | EcoR1 | |
| 7 | pGEX6P-2 | CuMTSE2 | Sal1, Not1 | |
| 8 | pGEX6P-2 | CuMTS3 | Non Treated | |
| 9 | pGEX6P-2 | CuMTS3 | EcoR1 | |
| 10 | pGEX6P-2 | CuMTS3 | Sal1, Not1 | |
| 11 | pGEX6P-2 | CuMTS62 | Non Treated | |
| 12 | pGEX6P-2 | CuMTS62 | EcoR1 | |
| 13 | pGEX6P-2 | CuMTS62 | Sal1, Not1 | |

June 12, 2014
KOBAYASHI
Transformation
| Host | Sample | |
|---|---|---|
| Vector | Insert | |
| BL21(DE3)pLysS | pGEX6P-2 | CuMTSE1 |
| BL21(DE3)pLysS | pGEX6P-2 | CuMTSE2 |
| BL21(DE3)pLysS | pGEX6P-2 | CuMTS3 |
| BL21(DE3)pLysS | pGEX6P-2 | CuMTS62 |
June 13, 2014
KOBAYASHI
Colony Isolation
Select 6 colonies per plate from 4 plates from June 12.
| Host | Sample | Section | |
|---|---|---|---|
| Vector | Insert | ||
| BL21(DE3)pLysS | pGEX6P-2 | CuMTSE1 | 1-a, 1-b |
| BL21(DE3)pLysS | pGEX6P-2 | CuMTSE2 | 2-a, 2-b |
| BL21(DE3)pLysS | pGEX6P-2 | CuMTS3 | 3-a, 3-b |
| BL21(DE3)pLysS | pGEX6P-2 | CuMTS62 | 6-a, 6-b |
June 14, 2014
ONISHI
Miniprep
| Host | Sample | |
|---|---|---|
| Vector | Insert | |
| 1-a | pGEX6P-2 | CuMTSE1 |
| 1-b | pGEX6P-2 | CuMTSE1 |
| 2-a | pGEX6P-2 | CuMTSE2 |
| 2-b | pGEX6P-2 | CuMTSE2 |
| 3-a | pGEX6P-2 | CuMTS3 |
| 3-b | pGEX6P-2 | CuMTS3 |
| 6-a | pGEX6P-2 | CuMTS62 |
| 6-b | pGEX6P-2 | CuMTS62 |
Miniprep
| Host | Sample | |
|---|---|---|
| Vector | Insert | |
| BL21(DE3) | pGEX6P-2 | CuMTSE1 |
| BL21(DE3) | pGEX6P-2 | CuMTSE2 |
| BL21(DE3) | pGEX6P-2 | CuMTS3 |
| BL21(DE3) | pGEX6P-2 | CuMTS62 |
Restriction Digest
| Host | Sample | Enzyme | Buffer | |
|---|---|---|---|---|
| Vector | Insert | |||
| 1-a | pGEX6P-2 | CuMTSE1 | EcoR1 | Buffer H |
| 1-b | pGEX6P-2 | CuMTSE1 | EcoR1 | Buffer H |
| 2-a | pGEX6P-2 | CuMTSE2 | EcoR1 | Buffer H |
| 2-b | pGEX6P-2 | CuMTSE2 | EcoR1 | Buffer H |
| 3-a | pGEX6P-2 | CuMTS3 | EcoR1 | Buffer H |
| 3-b | pGEX6P-2 | CuMTS3 | EcoR1 | Buffer H |
| 6-a | pGEX6P-2 | CuMTS62 | EcoR1 | Buffer H |
| 6-b | pGEX6P-2 | CuMTS62 | EcoR1 | Buffer H |
| BL21(DE3) | pGEX6P-2 | CuMTSE1 | EcoR1 | Buffer H |
| BL21(DE3) | pGEX6P-2 | CuMTSE2 | EcoR1 | Buffer H |
| BL21(DE3) | pGEX6P-2 | CuMTS3 | EcoR1 | Buffer H |
| BL21(DE3) | pGEX6P-2 | CuMTS62 | EcoR1 | Buffer H |
AGE
| Lane | Sample | Insert | Enzyme | Buffer | ||
|---|---|---|---|---|---|---|
| Marker | Host | Vector | ||||
| 1 | 1kb Ladder | |||||
| 2 | λDNA-HindⅢ | |||||
| 3 | 1-a | pGEX6P-2 | CuMTSE1 | EcoR1 | Buffer H | |
| 4 | 1-b | pGEX6P-2 | CuMTSE1 | EcoR1 | Buffer H | |
| 5 | 2-a | pGEX6P-2 | CuMTSE2 | EcoR1 | Buffer H | |
| 6 | 2-b | pGEX6P-2 | CuMTSE2 | EcoR1 | Buffer H | |
| 7 | 3-a | pGEX6P-2 | CuMTS3 | EcoR1 | Buffer H | |
| 8 | 3-b | pGEX6P-2 | CuMTS3 | EcoR1 | Buffer H | 9 | 6-a | pGEX6P-2 | CuMTS62 | EcoR1 | Buffer H |
| 10 | 6-b | pGEX6P-2 | CuMTS62 | EcoR1 | Buffer H | |
| 11 | BL21(DE3) | pGEX6P-2 | CuMTSE1 | EcoR1 | Buffer H | |
| 12 | BL21(DE3) | pGEX6P-2 | CuMTSE2 | EcoR1 | Buffer H | |
| 13 | BL21(DE3) | pGEX6P-2 | CuMTS3 | EcoR1 | Buffer H | |
| 14 | BL21(DE3) | pGEX6P-2 | CuMTS62 | EcoR1 | Buffer H | |
Apply λDNA-HindⅢ to compare and contrast AGE of July 14 and June 11.

June 16, 2014
YAMAJI
Preculture
| Host | Sample | |
|---|---|---|
| Vector | Insert | |
| BL21(DE3)pLysS | pGEX6P-2 | CuMTSE1 |
| BL21(DE3)pLysS | pGEX6P-2 | CuMTSE2 |
| BL21(DE3)pLysS | pGEX6P-2 | CuMTS3 |
| BL21(DE3)pLysS | pGEX6P-2 | CuMTS62 |
| BL21(DE3)pLysS | pGEX6P-2 | |
Main Culture
| Host | Sample | IPTG | |
|---|---|---|---|
| Vector | Insert | ||
| BL21(DE3)pLysS | pGEX6P-2 | CuMTSE1 | Induced |
| BL21(DE3)pLysS | pGEX6P-2 | CuMTSE2 | Induced |
| BL21(DE3)pLysS | pGEX6P-2 | CuMTS3 | Induced |
| BL21(DE3)pLysS | pGEX6P-2 | CuMTS62 | Induced |
| BL21(DE3)pLysS | pGEX6P-2 | Induced | |
| BL21(DE3)pLysS | pGEX6P-2 | CuMTSE1 | Non-induced |
| BL21(DE3)pLysS | pGEX6P-2 | CuMTSE2 | Non-induced |
| BL21(DE3)pLysS | pGEX6P-2 | CuMTS3 | Non-induced |
| BL21(DE3)pLysS | pGEX6P-2 | CuMTS62 | Non-induced |
| BL21(DE3)pLysS | pGEX6P-2 | Non-induced | |
June 18, 2014
YAMAJI
Preculture
| Host | Sample | |
|---|---|---|
| Vector | Insert | |
| BL21(DE3)pLysS | pGEX6P-2 | CuMTSE1 | BL21(DE3)pLysS | pGEX6P-2 | CuMTSE2 |
Main Culture
| Host | Sample | IPTG | |
|---|---|---|---|
| Vector | Insert | ||
| BL21(DE3)pLysS | pGEX6P-2 | Induced | |
| BL21(DE3)pLysS | pGEX6P-2 | CuMTSE1 | Induced |
| BL21(DE3)pLysS | pGEX6P-2 | CuMTSE2 | Induced |
| BL21(DE3)pLysS | pGEX6P-2 | Non-induced | |
| BL21(DE3)pLysS | pGEX6P-2 | CuMTSE1 | Non-induced |
| BL21(DE3)pLysS | pGEX6P-2 | CuMTSE2 | Non-induced |
Protein Extraction (E. coli)
| Host | Sample | IPTG | |
|---|---|---|---|
| Vector | Insert | ||
| BL21(DE3)pLysS | pGEX6P-2 | Induced | |
| BL21(DE3)pLysS | pGEX6P-2 | CuMTSE1 | Induced |
| BL21(DE3)pLysS | pGEX6P-2 | CuMTSE2 | Induced |
| BL21(DE3)pLysS | pGEX6P-2 | Non-induced | |
| BL21(DE3)pLysS | pGEX6P-2 | CuMTSE1 | Non-induced |
| BL21(DE3)pLysS | pGEX6P-2 | CuMTSE2 | Non-induced |
Preparations for SDS-PAGE
Add SDS sample buffer to protein extractions in a flask, incubate at 37℃ for 15 minutes and keep it at 4℃.

June 19, 2014
KITOMI
SDS-PAGE
| Host | Sample | IPTG | |
|---|---|---|---|
| Vector | Insert | ||
| BL21(DE3)pLysS | pGEX6P-2 | Induced | |
| BL21(DE3)pLysS | pGEX6P-2 | CuMTSE1 | Induced |
| BL21(DE3)pLysS | pGEX6P-2 | CuMTSE2 | Induced |
| BL21(DE3)pLysS | pGEX6P-2 | Non-induced | |
| BL21(DE3)pLysS | pGEX6P-2 | CuMTSE1 | Non-induced |
| BL21(DE3)pLysS | pGEX6P-2 | CuMTSE2 | Non-induced |
June 21, 2014
SOGA
Miniprep
| Host | Sample | |
|---|---|---|
| Vector | Insert | |
| BL21(DE3)pLysS | pGEX6P-2 | CuMTSE1 |
| BL21(DE3)pLysS | pGEX6P-2 | CuMTSE2 |
PCR
| Vector | Sample | DNA Polymerase | |
|---|---|---|---|
| Insert | Primer | ||
| pGEX6P-2 | CuMTSE1 | pGEX6P-2F1, pGEX6P-2R | KOD-FX-NEO |
| pGEX6P-2 | CuMTSE1 | pGEX6P-2F2, pGEX6P-2R | KOD-FX-NEO |
| pGEX6P-2 | CuMTSE2 | pGEX6P-2F1, pGEX6P-2R | KOD-FX-NEO |
| pGEX6P-2 | CuMTSE2 | pGEX6P-2F2, pGEX6P-2R | KOD-FX-NEO |
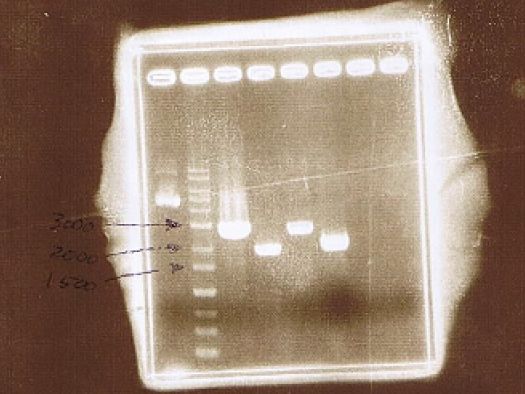
AGE
| Lane | Sample | Insert | Primer | ||
|---|---|---|---|---|---|
| Marker | Host | Vector | |||
| 1 | 1kb Ladder | ||||
| 2 | BY4247 | p427-TEF | |||
| 3 | pGEX6P-2 | CuMTSE1 | pGEX6P-2F1, pGEX6P-2R | ||
| 4 | pGEX6P-2 | CuMTSE1 | pGEX6P-2F2, pGEX6P-2R | ||
| 5 | pGEX6P-2 | CuMTSE2 | pGEX6P-2F1, pGEX6P-2R | ||
| 6 | pGEX6P-2 | CuMTSE2 | pGEX6P-2F2, pGEX6P-2R | ||
June 24, 2014
SOGA
Preculture
| Host | Sample | |
|---|---|---|
| Vector | Insert | |
| BL21(DE3)pLysS | ||
| BL21(DE3)pLysS | pGEX6P-2 | CuMTSE1 |
| BL21(DE3)pLysS | pGEX6P-2 | CuMTSE2 |
June 25, 2014
KOBAYASHI
Cultivate following samples in Big Scale at 30℃ overnight in shaken culture.
| Host | Sample | |
|---|---|---|
| Vector | Insert | |
| BL21(DE3)pLysS | ||
| BL21(DE3)pLysS | pGEX6P-2 | CuMTSE1 |
| BL21(DE3)pLysS | pGEX6P-2 | CuMTSE2 |
DNA Refinement 1
| Sample | |
|---|---|
| DNA | Primer |
| CuMTSE1 | pGEX6P-2F1, pGEX6P-2R |
| CuMTSE1 | pGEX6P-2F2, pGEX6P-2R |
Restriction Digest
| Vector | Sample | Enzyme | Buffer | |
|---|---|---|---|---|
| DNA | Primer | |||
| CuMTSE1 | pGEX6P-2F1, pGEX6P-2R | Spe1, Xho1 | Buffer H | |
| CuMTSE1 | pGEX6P-2F2, pGEX6P-2R | Spe1, Xho1 | Buffer H | |
| p427-TEF | Spe1, Xho1 | Buffer H | ||
DNA Refinement 2
| Vector | Sample | Enzyme | |
|---|---|---|---|
| DNA | Primer | ||
| CuMTSE1 | pGEX6P-2F1, pGEX6P-2R | Spe1, Xho1 | |
| CuMTSE1 | pGEX6P-2F2, pGEX6P-2R | Spe1, Xho1 | |
| p427-TEF | Spe1, Xho1 | ||
Main Culture
| Host | Sample | IPTG | |
|---|---|---|---|
| Vector | Insert | ||
| BL21(DE3)pLysS | pGEX6P-2 | Induced | |
| BL21(DE3)pLysS | pGEX6P-2 | CuMTSE1 | Induced |
| BL21(DE3)pLysS | pGEX6P-2 | CuMTSE2 | Induced |
| BL21(DE3)pLysS | pGEX6P-2 | Non-induced | |
| BL21(DE3)pLysS | pGEX6P-2 | CuMTSE1 | Non-induced |
| BL21(DE3)pLysS | pGEX6P-2 | CuMTSE2 | Non-induced |

Ligation
| Sample | ||
|---|---|---|
| Vector | DNA | Primer |
| p427-TEF | CuMTSE1 | pGEX6P-2F1, pGEX6P-2R |
| p427-TEF | CuMTSE1 | pGEX6P-2F2, pGEX6P-2R |
| p427-TEF | CuMTSE2 | pGEX6P-2F1, pGEX6P-2R |
| p427-TEF | CuMTSE2 | pGEX6P-2F2, pGEX6P-2R |
Transformation
| Host | Sample | ||
|---|---|---|---|
| Vector | Insert | Primer | |
| DH5α | p427-TEF | CuMTSE1 | pGEX6P-2F1, pGEX6P-2R |
| DH5α | p427-TEF | CuMTSE1 | pGEX6P-2F2, pGEX6P-2R |
| DH5α | p427-TEF | CuMTSE2 | pGEX6P-2F1, pGEX6P-2R |
| DH5α | p427-TEF | CuMTSE2 | pGEX6P-2F2, pGEX6P-2R |
June 26, 2014
SAWANO
Colony Isolation
Isolate each colony from the plates which were inoculated on June 25.
| Host | Sample | Section | ||
|---|---|---|---|---|
| Vector | Insert | Primer | ||
| DH5α | p427-TEF | CuMTSE1 | pGEX6P-2F1, pGEX6P-2R | 1~4 |
| DH5α | p427-TEF | CuMTSE1 | pGEX6P-2F2, pGEX6P-2R | a~h |
| DH5α | p427-TEF | CuMTSE1 | pGEX6P-2F2, pGEX6P-2R | ア~タ |
June 27, 2014
SHIMADA
Colony Sweep
| Lane | Sample | ||
|---|---|---|---|
| Host | Vector | Insert | |
| 1 | BL21(DE3)pLysS | pGEX6P-2 | CuMTSE1 |
| 2 | 1 | ||
| 3 | 2 | ||
| 4 | 3 | ||
| 5 | 4 | ||
| 6 | a | ||
| 7 | b | ||
| 8 | c | ||
| 9 | d | ||
| 10 | e | ||
| 11 | f | ||
| 12 | g | ||
| 13 | h | ||

Colony Sweep
| Lane | Sample | ||
|---|---|---|---|
| Host | Vector | Insert | |
| 1 | BL21(DE3)pLysS | pGEX6P-2 | CuMTSE1 |
| 2 | ア | ||
| 3 | イ | ||
| 4 | ウ | ||
| 5 | エ | ||
| 6 | オ | ||
| 7 | カ | ||
| 8 | キ | ||
| 9 | ク | ||
| 10 | ケ | ||
| 11 | コ | ||
| 12 | サ | ||
| 13 | シ | ||
| 14 | ス | ||
| 15 | セ | ||
| 16 | ソ | ||
| 17 | タ | ||

Identified targeted genes in 4, c, h, イ, カ, セ
Miniprep
| Sample | |
|---|---|
| Host | |
| 4 | |
| C | |
| H | |
| イ | |
| カ | |
| セ | |
Restriction Digest
| Sample | Enzyme | Buffer |
|---|---|---|
| Host | ||
| 4 | Spe1, Xho1 | Buffer H |
| C | Spe1, Xho1 | Buffer H |
| H | Spe1, Xho1 | Buffer H |
| イ | Spe1, Xho1 | Buffer H |
| カ | Spe1, Xho1 | Buffer H |
AGE
| Lane | Sample | Primer | Enzyme | ||
|---|---|---|---|---|---|
| Marker | Host | Insert | |||
| 1 | 1kb Ladder | ||||
| 2 | CuMTSE1 | pGEX6P-2F1, pGEX6P-2R | |||
| 3 | 4 | Spe1, Xho1 | |||
| 4 | 4 | Spe1, Xho1 | |||
| 5 | 4 | Non Treated | |||
| 6 | CuMTSE1 | pGEX6P-2F2, pGEX6P-2R | |||
| 7 | c | Spe1, Xho1 | |||
| 8 | c | Spe1, Xho1 | |||
| 9 | c | Non Treated | |||
| 10 | h | Spe1, Xho1 | |||
| 11 | h | Spe1, Xho1 | |||
| 12 | h | Non Treated | |||

Transformation (electroportation)
| Host | Sample | ||
|---|---|---|---|
| Vector | Insert | Primer | |
| BY4247 | p427-TEF | CuMTSE1 | pGEX6P-2F1, pGEX6P-2R |
| BY4247 | p427-TEF | CuMTSE1 | pGEX6P-2F2, pGEX6P-2R |
June 30, 2014
SOGA
Inoculation
Divide each of 2 plates into 8 sections and inoculate following samples on them.
| Host | Sample | Section | ||
|---|---|---|---|---|
| Vector | Insert | Primer | ||
| BY4247 | p427-TEF | CuMTSE1 | pGEX6P-2F1, pGEX6P-2R | A~H |
| BY4247 | p427-TEF | CuMTSE1 | pGEX6P-2F2, pGEX6P-2R | 一~ハ |
July
July 1, 2014
KOBAYASHI
Protein Extraction (S. cerevisiae)
| Host | Sample | ||
|---|---|---|---|
| Vector | Insert | Primer | |
| BY4247 | p427-TEF | CuMTSE1 | pGEX6P-2F1, pGEX6P-2R |
| BY4247 | p427-TEF | CuMTSE1 | pGEX6P-2F2, pGEX6P-2R |
July 2, 2014
ONISHI
Miniprep
| Host | Sample | ||
|---|---|---|---|
| Vector | Insert | Primer | |
| BY4247 | p427-TEF | ||
| BY4247 | p427-TEF | CuMTSE1 | pGEX6P-2F1, pGEX6P-2R |
| BY4247 | p427-TEF | CuMTSE1 | pGEX6P-2F2, pGEX6P-2R |
Restriction Digest
| Sample | Enzyme | Buffer | ||
|---|---|---|---|---|
| Vector | Insert | Primer | ||
| p427-TEF | CuMTSE1 | EcoR1 | Buffer H | |
| p427-TEF | CuMTSE1 | pGEX6P-2F1, pGEX6P-2R | EcoR1 | Buffer H |
| p427-TEF | CuMTSE1 | pGEX6P-2F2, pGEX6P-2R | EcoR1 | Buffer H |
| p427-TEF | CuMTSE1 | Sac1, Kpn1 | Buffer L | |
| p427-TEF | CuMTSE1 | pGEX6P-2F1, pGEX6P-2R | Sac1, Kpn1 | Buffer L |
| p427-TEF | CuMTSE1 | pGEX6P-2F2, pGEX6P-2R | Sac1, Kpn1 | Buffer L |
AGE
| Lane | Sample | Enzyme | |||
|---|---|---|---|---|---|
| Marker | Vector | Insert | Primer | ||
| 1 | p427-TEF | Non Treated | |||
| 2 | p427-TEF | CuMTSE1 | pGEX6P-2F1, pGEX6P-2R | Non Treated | |
| 3 | p427-TEF | CuMTSE1 | pGEX6P-2F2, pGEX6P-2R | Non Treated | |
| 4 | 1kb Ladder | ||||
| 5 | p427-TEF | EcoR1 | |||
| 6 | p427-TEF | CuMTSE1 | pGEX6P-2F1, pGEX6P-2R | EcoR1 | |
| 7 | p427-TEF | CuMTSE1 | pGEX6P-2F2, pGEX6P-2R | EcoR1 | |
| 8 | 1kb Ladder | ||||
| 9 | p427-TEF | Kpn1 | |||
| 10 | p427-TEF | CuMTSE1 | pGEX6P-2F1, pGEX6P-2R | Kpn1 | |
| 11 | p427-TEF | CuMTSE1 | pGEX6P-2F2, pGEX6P-2R | Kpn1 | |
| 12 | 1kb Ladder | ||||
| 13 | p427-TEF | Sac1 | |||
| 14 | p427-TEF | CuMTSE1 | pGEX6P-2F1, pGEX6P-2R | Sac1 | |
| 15 | p427-TEF | CuMTSE1 | pGEX6P-2F2, pGEX6P-2R | Sac1 | |

Restriction Digest
| Sample | Enzyme | Buffer | ||
|---|---|---|---|---|
| Vector | Insert | Primer | ||
| p427-TEF | CuMTSE1 | EcoR1 | Buffer H | |
| p427-TEF | CuMTSE1 | pGEX6P-2F1, pGEX6P-2R | EcoR1 | Buffer H |
| p427-TEF | CuMTSE1 | pGEX6P-2F2, pGEX6P-2R | EcoR1 | Buffer H |
| p427-TEF | CuMTSE1 | Sac1 | Buffer L | |
| p427-TEF | CuMTSE1 | pGEX6P-2F1, pGEX6P-2R | Sac1 | Buffer L |
| p427-TEF | CuMTSE1 | pGEX6P-2F2, pGEX6P-2R | Sac1 | Buffer L |
| p427-TEF | CuMTSE1 | Kpn1 | Buffer L | |
| p427-TEF | CuMTSE1 | pGEX6P-2F1, pGEX6P-2R | Kpn1 | Buffer L |
| p427-TEF | CuMTSE1 | pGEX6P-2F2, pGEX6P-2R | Kpn1 | Buffer L |
| p427-TEF | CuMTSE1 | Spe1, Xho1 | Buffer H | |
| p427-TEF | CuMTSE1 | pGEX6P-2F1, pGEX6P-2R | Spe1, Xho1 | Buffer H |
| p427-TEF | CuMTSE1 | pGEX6P-2F2, pGEX6P-2R | Spe1, Xho1 | Buffer H |
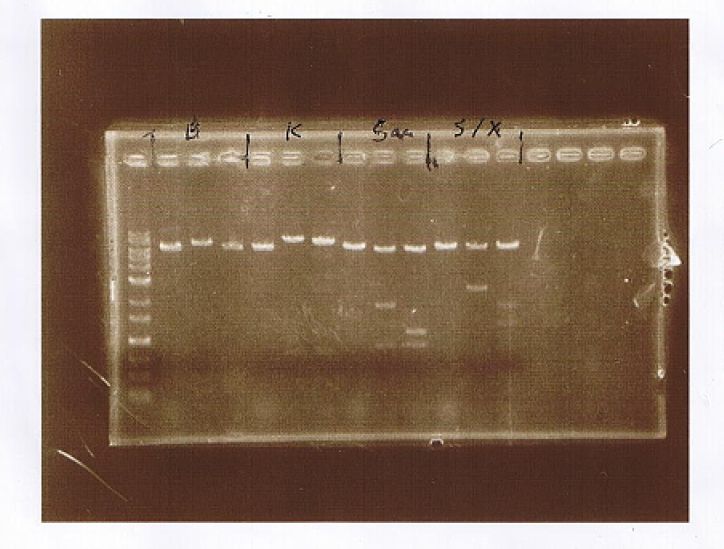
AGE
| Lane | Sample | Enzyme | |||
|---|---|---|---|---|---|
| Marker | Vector | Insert | Primer | ||
| 1 | 1kb Ladder | ||||
| 2 | p427-TEF | CuMTSE1 | EcoR1 | ||
| 3 | p427-TEF | CuMTSE1 | pGEX6P-2F1, pGEX6P-2R | EcoR1 | |
| 4 | p427-TEF | CuMTSE1 | pGEX6P-2F2, pGEX6P-2R | EcoR1 | |
| 5 | p427-TEF | CuMTSE1 | Kpn1 | ||
| 6 | p427-TEF | CuMTSE1 | pGEX6P-2F1, pGEX6P-2R | Kpn1 | |
| 7 | p427-TEF | CuMTSE1 | pGEX6P-2F2, pGEX6P-2R | Kpn1 | |
| 8 | p427-TEF | CuMTSE1 | Sac1 | ||
| 9 | p427-TEF | CuMTSE1 | pGEX6P-2F1, pGEX6P-2R | Sac1 | |
| 10 | p427-TEF | CuMTSE1 | pGEX6P-2F2, pGEX6P-2R | Sac1 | |
| 11 | p427-TEF | CuMTSE1 | Spe1, Xho1 | ||
| 12 | p427-TEF | CuMTSE1 | pGEX6P-2F1, pGEX6P-2R | Spe1, Xho1 | |
| 13 | p427-TEF | CuMTSE1 | pGEX6P-2F2, pGEX6P-2R | Spe1, Xho1 | |
July 3, 2014
OKAMOTO
PCR
| Sample | DNA Polymerase | ||
|---|---|---|---|
| Vector | Insert | Primer | |
| pGEX6P-2 | CuMTSE2 | pGEX6P-2F1, pGEX6P-2R | KOD-FX-NEO |
| pGEX6P-2 | CuMTSE2 | pGEX6P-2F2, pGEX6P-2R | KOD-FX-NEO |
| pGEX6P-2 | CuMTS3 | pGEX6P-2F1, pGEX6P-2R | KOD-FX-NEO |
| pGEX6P-2 | CuMTS3 | pGEX6P-2F2, pGEX6P-2R | KOD-FX-NEO |
| pGEX6P-2 | CuMTS62 | pGEX6P-2F1, pGEX6P-2R | KOD-FX-NEO |
| pGEX6P-2 | CuMTS62 | pGEX6P-2F2, pGEX6P-2R | KOD-FX-NEO |
Protein Extraction (S. cerevisiae)
| Host | Sample | ||
|---|---|---|---|
| Vector | Insert | Primer | |
| BY4247 | p427-TEF | ||
| A | p427-TEF | CuMTSE1 | pGEX6P-2F1, pGEX6P-2R |
| 六 | p427-TEF | CuMTSE1 | pGEX6P-2F2, pGEX6P-2R |
July 4, 2014
SHIMADA
AGE
| Lane | Sample/Marker | |||
|---|---|---|---|---|
| Marker | Vector | Insert | Primer | |
| 1 | 1kb Ladder | |||
| 2 | pGEX6P-2 | CuMTSE2 | pGEX6P-2F1, pGEX6P-2R | |
| 3 | pGEX6P-2 | CuMTSE2 | pGEX6P-2F2, pGEX6P-2R | |
| 4 | pGEX6P-2 | CuMTS3 | pGEX6P-2F1, pGEX6P-2R | |
| 5 | pGEX6P-2 | CuMTS3 | pGEX6P-2F2, pGEX6P-2R | |
| 6 | pGEX6P-2 | CuMTS62 | pGEX6P-2F1, pGEX6P-2R | |
| 7 | pGEX6P-2 | CuMTS62 | pGEX6P-2F2, pGEX6P-2R | |

PCR
| Sample | DNA Polymerase | ||
|---|---|---|---|
| Vector | Insert | Primer | |
| pGEX6P-2 | CuMTSE2 | pGEX6P-2F1, pGEX6P-2R | KOD-FX-NEO |
| pGEX6P-2 | CuMTSE2 | pGEX6P-2F2, pGEX6P-2R | KOD-FX-NEO |
| pGEX6P-2 | CuMTS3 | pGEX6P-2F1, pGEX6P-2R | KOD-FX-NEO |
| pGEX6P-2 | CuMTS3 | pGEX6P-2F2, pGEX6P-2R | KOD-FX-NEO |
| pGEX6P-2 | CuMTS62 | pGEX6P-2F1, pGEX6P-2R | KOD-FX-NEO |
| pGEX6P-2 | CuMTS62 | pGEX6P-2F2, pGEX6P-2R | KOD-FX-NEO |
AGE
| Lane | Sample | |||
|---|---|---|---|---|
| Marker | Vector | Insert | Primer | |
| 1 | 1kb Ladder | |||
| 2 | pGEX6P-2 | CuMTSE2 | pGEX6P-2F1, pGEX6P-2R | |
| 3 | pGEX6P-2 | 1kb Ladder+K83:N91 | pGEX6P-2F2, pGEX6P-2R | |
| 4 | pGEX6P-2 | CuMTS3 | pGEX6P-2F1, pGEX6P-2R | |
| 5 | pGEX6P-2 | CuMTS3 | pGEX6P-2F2, pGEX6P-2R | |
| 6 | pGEX6P-2 | CuMTS62 | pGEX6P-2F1, pGEX6P-2R | |
| 7 | pGEX6P-2 | CuMTS62 | pGEX6P-2F2, pGEX6P-2R | |

July 5, 2014
KOBAYASHI
SDS-PAGE
| Host | Sample | ||
|---|---|---|---|
| Vector | Insert | Primer | |
| BL21(DE3)pLysS | pGEX6P-2 | ||
| 4 | pGEX6P-2 | CuMTSE1 | pGEX6P-2F1, pGEX6P-2R |
| C | pGEX6P-2 | CuMTSE2 | pGEX6P-2F2, pGEX6P-2R |
| BY4247 | p427-TEF | ||
| A | p427-TEF | CuMTSE1 | pGEX6P-2F1, pGEX6P-2R |
| 六 | p427-TEF | CuMTSE1 | pGEX6P-2F2, pGEX6P-2R |
July 6, 2014
SOGA
DNA Refinement 1
| Sample | |
|---|---|
| DNA | Primer |
| CuMTSE2 | pGEX6P-2F1, pGEX6P-2R |
| CuMTSE2 | pGEX6P-2F2, pGEX6P-2R |
| CuMTS3 | pGEX6P-2F1, pGEX6P-2R |
| CuMTS62 | pGEX6P-2F1, pGEX6P-2R |
Restriction Digest
| Vector | Sample | Enzyme | Buffer | |
|---|---|---|---|---|
| DNA | Primer | |||
| CuMTSE2 | pGEX6P-2F1, pGEX6P-2R | Spe1, Xho1 | Fast digest buffer | |
| CuMTSE2 | pGEX6P-2F2, pGEX6P-2R | Spe1, Xho1 | Fast digest buffer | |
| CuMTS3 | pGEX6P-2F1, pGEX6P-2R | Spe1, Xho1 | Fast digest buffer | CuMTS62 | pGEX6P-2F1, pGEX6P-2R | Spe1, Xho1 | Fast digest buffer | p427-TEF | Spe1, Xho1 | Fast digest buffer |
DNA Refinement 2
| Sample | Enzyme | |
|---|---|---|
| DNA | Primer | |
| CuMTSE2 | pGEX6P-2F1, pGEX6P-2R | Spe1, Xho1 |
| CuMTSE2 | pGEX6P-2F2, pGEX6P-2R | Spe1, Xho1 |
Ligation
| Vector | Sample | Enzyme | |
|---|---|---|---|
| DNA | Primer | ||
| p427-TEF | CuMTSE2 | pGEX6P-2F1, pGEX6P-2R | Spe1, Xho1 |
| p427-TEF | CuMTSE2 | pGEX6P-2F2, pGEX6P-2R | Spe1, Xho1 |
DNA ligase: DNA Ligation Kit ‹Mighty Mix› (TAKARA BIO INC.)
Transformation (E. coli)
| Host | Sample | Enzyme | ||
|---|---|---|---|---|
| Vector | Insert | Primer | ||
| DH5α | p427-TEF | CuMTSE2 | pGEX6P-2F1, pGEX6P-2R | Spe1, Xho1 |
| DH5α | p427-TEF | CuMTSE2 | pGEX6P-2F2, pGEX6P-2R | Spe1, Xho1 |
| DH5α | p427-TEF | CuMTS3 | pGEX6P-2F1, pGEX6P-2R | Spe1, Xho1 |
| DH5α | p427-TEF | CuMTS62 | pGEX6P-2F1, pGEX6P-2R | Spe1, Xho1 |
July 7, 2014
KOBAYASHI
Colony Isolation
Isolate each colony from the plates which were inoculated on July 6.
| Host | Sample | Section | ||
|---|---|---|---|---|
| Vector | Insert | Primer | ||
| DH5α | p427-TEF | CuMTSE2 | pGEX6P-2F1, pGEX6P-2R | 1~16 |
| DH5α | p427-TEF | CuMTS3 | pGEX6P-2F1, pGEX6P-2R | ア~エ |
| DH5α | p427-TEF | CuMTS62 | pGEX6P-2F1, pGEX6P-2R | a~p |
Transformation (E. coli)
| Host | Sample | Enzyme | ||
|---|---|---|---|---|
| Vector | Insert | Primer | ||
| DH5α | p427-TEF | CuMTSE2 | pGEX6P-2F2, pGEX6P-2R | Spe1, Xho1 |
July 8, 2014
OKAMOTO
Colony Sweep
| Lane | Sample | |
|---|---|---|
| Host | ||
| 1 | DH5α | p427-TEF |
| 2 | a | |
| 3 | b | |
| 4 | c | |
| 5 | d | |
| 6 | e | |
| 7 | f | |
| 8 | g | |
| 9 | h | |
| 10 | i | |
| 11 | j | |
| 12 | k | |
| 13 | l | |
| 14 | m | |
| 15 | n | |
| 16 | o | |
| 17 | p | |

Colony Sweep
| Lane | Sample | |
|---|---|---|
| Host | ||
| 1 | DH5α | p427-TEF |
| 2 | ア | |
| 3 | イ | |
| 4 | ウ | |
| 5 | エ | |

Colony Sweep
| Lane | Sample | |
|---|---|---|
| Host | ||
| 1 | DH5α | p427-TEF |
| 2 | 1 | |
| 3 | 2 | |
| 4 | 3 | |
| 5 | 4 | |
| 6 | 5 | |
| 7 | 6 | |
| 8 | 7 | |
| 9 | 8 | |
| 10 | 9 | |
| 11 | 10 | |
| 12 | 11 | |
| 13 | 12 | |
| 14 | 13 | |
| 15 | 14 | |
| 16 | 15 | |
| 17 | 16 | |

July 9, 2014
OKAMOTO
Main Culture
| Sample |
|---|
| Host |
| d |
| h |
| l |
| ア |
| イ |
| エ |
Mini Prep
| Sample |
|---|
| Host |
| D |
| H |
| L |
| ア |
| イ |
| エ |
Restriction Digest
| Vector | Sample | Enzyme | Buffer | |
|---|---|---|---|---|
| Insert | Primer | |||
| p427-TEF(d) | CuMTS3 | pGEX6P-2F1, pGEX6P-2R | Spe1, Xho1 | Fast digest buffer |
| p427-TEF(h) | CuMTS3 | pGEX6P-2F1, pGEX6P-2R | Spe1, Xho1 | Fast digest buffer |
| p427-TEF(l) | CuMTS3 | pGEX6P-2F1, pGEX6P-2R | Spe1, Xho1 | Fast digest buffer |
| p427-TEF(ア) | CuMTS62 | pGEX6P-2F1, pGEX6P-2R | Spe1, Xho1 | Fast digest buffer |
| p427-TEF(イ) | CuMTS62 | pGEX6P-2F1, pGEX6P-2R | Spe1, Xho1 | Fast digest buffer |
| p427-TEF(エ) | CuMTS62 | pGEX6P-2F1, pGEX6P-2R | Spe1, Xho1 | Fast digest buffer |
AGE
| Lane | Sample | Enzyme | ||
|---|---|---|---|---|
| Vector | Insert | Primer | ||
| 1 | p427-TEF(ア) | CuMTS3 | pGEX6P-2F1, pGEX6P-2R | Non Treated |
| 2 | p427-TEF(ア) | CuMTS3 | pGEX6P-2F1, pGEX6P-2R | Spe1, Xho1 |
| 3 | p427-TEF(イ) | CuMTS3 | pGEX6P-2F1, pGEX6P-2R | Non Treated |
| 4 | p427-TEF(イ) | CuMTS3 | pGEX6P-2F1, pGEX6P-2R | Spe1, Xho1 |
| 5 | p427-TEF(エ) | CuMTS3 | pGEX6P-2F1, pGEX6P-2R | Non Treated |
| 6 | p427-TEF(エ) | CuMTS3 | pGEX6P-2F1, pGEX6P-2R | Spe1, Xho1 |
| 7 | p427-TEF(d) | CuMTS62 | pGEX6P-2F1, pGEX6P-2R | Non Treated |
| 8 | p427-TEF(d) | CuMTS62 | pGEX6P-2F1, pGEX6P-2R | Spe1, Xho1 |
| 9 | p427-TEF(h) | CuMTS62 | pGEX6P-2F1, pGEX6P-2R | Non Treated |
| 10 | p427-TEF(h) | CuMTS62 | pGEX6P-2F1, pGEX6P-2R | Spe1, Xho1 |
| 11 | p427-TEF(l) | CuMTS62 | pGEX6P-2F1, pGEX6P-2R | Non Treated |
| 12 | p427-TEF(l) | CuMTS62 | pGEX6P-2F1, pGEX6P-2R | Spe1, Xho1 |

August
August 19, 2014
KOBAYASHI
Conduct mutagenesis to remove an illegal restriction site (EcoR1)
InversePCR
| Sample | |||
|---|---|---|---|
| Vector | Insert | Primer | DNA polymerase |
| pGEX6P-2 | CuMTS3 | Terpinene3-mutagenesis-FP, Terpinene3-mutagenesis-RP | KOD-Plus |
August 20, 2014
KOBAYASHI
Restart mutagenesis from KOD -Plus- Mutagenesis Kit protocol No.3 to obtain plasmid (muta-CuMTS3 in pGEX-6P-2)
Transformation
| Sample | |||
|---|---|---|---|
| Host | Vector | Insert | Primer |
| DH5α | pGEX6P-2 | muta-CuMTS3 | Terpinene3-mutagenesis-FP and Terpinene3-mutagenesis-RP |
August 21, 2014
KOBAYASHI
Colony Isolation
Isolate each colony from two plates which were inoculated overnight
| Sample | Section | |||
|---|---|---|---|---|
| Host | Vector | Insert | Primer | |
| DH5α | pSB1C3 | muta-CuMTS3 | Terpinene3-mutagenesis-FP, Terpinene3-mutagenesis-RP |
あ-み |
August 22, 2014
KOBAYASHI
Restriction Digest
| Sample | Enzyme | Buffer | ||
|---|---|---|---|---|
| Host | Vector | Insert | ||
| DH5α あ | pGEX6P-2 | muta-CuMTS3 | Sal1 | Fast Digest buffer |
| DH5α か | pGEX6P-2 | muta-CuMTS3 | Sal1 | Fast Digest buffer |
| DH5α さ | pGEX6P-2 | muta-CuMTS3 | Sal1 | Fast Digest buffer |
| DH5α た | pGEX6P-2 | muta-CuMTS3 | Sal1 | Fast Digest buffer |
AGE
| Lane | Sample | Enzyme | |||
|---|---|---|---|---|---|
| Marker | Host | Vector | Insert | ||
| 1 | 1kb Ladder | ||||
| 2 | DH5α あ | pGEX6P-2 | muta-CuMTS3 | Sal1 | |
| 3 | DH5α か | pGEX6P-2 | muta-CuMTS3 | Sal1 | |
| 4 | DH5α さ | pGEX6P-2 | muta-CuMTS3 | Sal1 | |
| 5 | DH5α た | pGEX6P-2 | muta-CuMTS3 | Sal1 | |
Restriction Digest
| Sample | Enzyme | Buffer | ||
|---|---|---|---|---|
| Host | Vector | Insert | ||
| DH5α か | pGEX6P-2 | CuMTS3 | EcoR1 | Fast Digest buffer |
| DH5α か | pGEX6P-2 | muta-CuMTS3 | EcoR1 | Fast Digest buffer |
AGE
| Lane | Sample | Enzyme | |||
|---|---|---|---|---|---|
| Marker | Host | Vector | Insert | ||
| 1 | 1kb Ladder | ||||
| 2 | DH5α か | pGEX6P-2 | CuMTS3 | EcoR1 | |
| 3 | DH5α か | pGEX6P-2 | muta-CuMTS3 | EcoR1 | |
 An illegal EcoR1 restriction site was removed.
An illegal EcoR1 restriction site was removed.PCR
| Sample | DNA polymerase | ||
|---|---|---|---|
| Vector | Insert | Primer | |
| か pGEX6P-2 | muta-CuMTS3 | terpinene3-prefix and terpinene3-suffix | KOD-FX-NEO-Plus |
AGE
| Lane | Sample | DNA polymerase | |||
|---|---|---|---|---|---|
| Marker | Vector | Insert | Primer | ||
| 1 | 1kb Ladder | ||||
| 2 | か pGEX6P-2 | muta-CuMTS3 | terpinene3-prefix and terpinene3-suffix | KOD-FX-NEO-Plus | |

August 30, 2014
KOBAYASHIPCR
| Sample | DNA polymerase | ||
|---|---|---|---|
| Vector | Insert | Primer | |
| か pGEX6P-2 | muta-CuMTS3 | terpinene3-prefix-Xba1 and terpinene3-suffix-Spe1 | Taq |
AGE
| Lane | Sample | |||
|---|---|---|---|---|
| Marker | Vector | Insert | Primer | |
| 1 | 1kb Ladder | |||
| 2 | か pGEX6P-2 | muta-CuMTS3 | terpinene3-prefix-Xba1 and terpinene3-suffix-Spe1 | |
 Made total amount of 295 μL of PCR samples.
Made total amount of 295 μL of PCR samples.
September
September 18, 2014
KOBAYASHIMiniprep
| Sample | ||
|---|---|---|
| Host | Vector | Insert |
| DH5α | pSB1C3 | BBa_J04450 |
| DH5α | pSB1C3 | BBa_J04450 |
| DH5α | pSB1C3 | BBa_J04450 |
Restriction Digest
| Sample | Buffer | ||
|---|---|---|---|
| Vector | Insert | Enzyme | |
| pSB1C3 | BBa_J04450 | EcoR1, Pst1 | Buffer H |
| pSB1C3 | BBa_J04450 | Xba1, Spe1 | Fast Digest buffer |
| pSB1C3 | BBa_J04450 | ||
AGE
| Lane | Sample | Enzyme | |||
|---|---|---|---|---|---|
| Marker | Host | Vector | Insert | ||
| 1 | 1kb Ladder | ||||
| 2 | DH5α | pSB1C3 | BBa_J04450 | EcoR1, Pst1 | |
| 3 | DH5α | pSB1C3 | BBa_J04450 | Xba1, Spe1 | |
| 4 | DH5α | pSB1C3 | BBa_J04450 | ||

PCR
| Sample | DNA polymerase | ||
|---|---|---|---|
| Vector | Insert | Primer | |
| か pGEX6P-2 | muta-CuMTS3 | terpinene3-prefix-Xba1 and terpinene3-suffix-Spe1 | Taq |
DNA Refinement 1
| Sample | |
|---|---|
| Insert | Primer |
| muta-CuMTS3 | terpinene3-prefix-Xba1 and terpinene3-suffix-Spe1 |
Restriction Digest
| Sample | Enzyme | Buffer | ||
|---|---|---|---|---|
| Vector | Insert | Primer | ||
| pSB1C3 | Xba1, Spe1 | Fast Digest | ||
| か pGEX6P-2 | muta-CuMTS3 | terpinene3-prefix-Xba1 and terpinene3-suffix-Spe1 | Xba1, Spe1 | Fast Digest |
DNA Refinement 2
| Sample | |
|---|---|
| Insert | Primer |
| muta-CuMTS3 | terpinene3-prefix-Xba1 and terpinene3-suffix-Spe1 |
Ligation
| Sample | Enzyme | ||
|---|---|---|---|
| Vector | DNA | Primer | |
| pSB1C3 | muta-CuMTS3 | terpinene3-prefix-Xba1 and terpinene3-suffix-Spe1 | Spe1, Xba1 |
Transformation
| Sample | |||
|---|---|---|---|
| Host | Vector | Insert | Primer |
| DH5α | pSB1C3 | muta-CuMTS3 | terpinene3-prefix-Xba1 and terpinene3-suffix-Spe1 |
September 19, 2014
KOBAYASHIColony Isolation
Isolate each colony from the plates which were inoculated overnight.
| Sample | Section | |||
|---|---|---|---|---|
| Host | Vector | Insert | Primer | |
| DH5α | pSB1C3 | muta-CuMTS3 | terpinene3-prefix-Xba1 and terpinene3-suffix-Spe1 | A-P |
| DH5α | pSB1C3 | muta-CuMTS3 | terpinene3-prefix-Xba1 and terpinene3-suffix-Spe1 | ア-タ |
Colony Sweep
| Lane | Sample | ||
|---|---|---|---|
| Host | Vector | Insert | |
| 1 | DH5α | pSB1C3 | muta-CuMTS3 |
| 2 | ア | ||
| 3 | イ | ||
| 4 | ウ | ||
| 5 | エ | ||
| 6 | オ | ||
| 7 | カ | ||
| 8 | キ | ||
| 9 | ク | ||
| 10 | ケ | ||
| 11 | コ | ||
| 12 | サ | ||
| 13 | シ | ||
| 14 | ス | ||
| 15 | セ | ||
| 16 | ソ | ||
| 17 | タ | ||
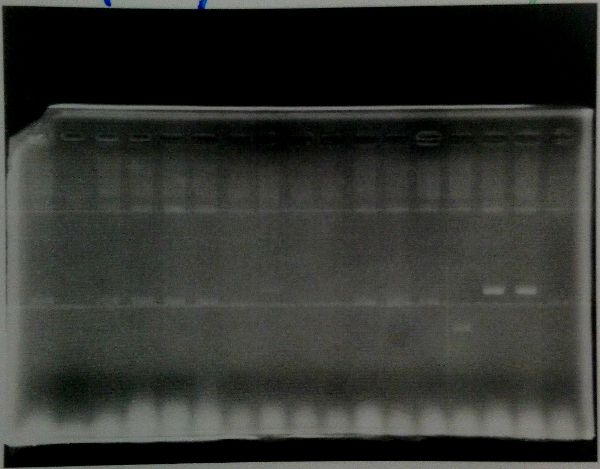
Targeted colonies were identified in セ and ソ.
Colony Sweep
| Lane | Sample | ||
|---|---|---|---|
| Host | Vector | Insert | |
| 1 | DH5α | pSB1C3 | muta-CuMTS3 |
| 2 | A | ||
| 3 | B | ||
| 4 | C | ||
| 5 | D | ||
| 6 | E | ||
| 7 | F | ||
| 8 | G | ||
| 9 | H | ||
| 10 | I | ||
| 11 | J | ||
| 12 | K | ||
| 13 | L | ||
| 14 | M | ||
| 15 | N | ||
| 16 | O | ||
| 17 | P | ||

Targeted colonies were identified in colony N and F.
Select セ, ソ, N, F
Main Culture
| Sample | |||
|---|---|---|---|
| Host | Vector | Insert | Primer |
| DH5α(N) | pSB1C3 | muta-CuMTS3 | ---------- |
| DH5α(F) | pSB1C3 | muta-CuMTS3 | ---------- |
| DH5α(セ) | pSB1C3 | muta-CuMTS3 | ---------- |
| DH5α(ソ) | pSB1C3 | muta-CuMTS3 | ---------- |
Cultivate these samples in 3mL of LB medium with ChlPhe at 30°C overnight.
September 20, 2014
KOBAYASHIMiniprep
| Sample | ||
|---|---|---|
| Host | Vector | Primer |
| DH5α(N) | pSB1C3 | muta-CuMTS3 |
| DH5α(F) | pSB1C3 | muta-CuMTS3 |
| DH5α(セ) | pSB1C3 | muta-CuMTS3 |
| DH5α(ソ) | pSB1C3 | muta-CuMTS3 |
Enzyme: E/P and Xba1
Restriction Digest
| Sample | Enzyme | Buffer | |||
|---|---|---|---|---|---|
| Marker | Host | Vector | Insert | ||
| pSB1C3 | |||||
| DH5α (セ) | pSB1C3 | muta-CuMTS3 | |||
| DH5α (ソ) | pSB1C3 | muta-CuMTS3 | |||
| DH5α (F) | pSB1C3 | muta-CuMTS3 | |||
| DH5α (N) | pSB1C3 | muta-CuMTS3 | |||
| DH5α (セ) | pSB1C3 | EcoR1, Pst1 | Buffer H | ||
| DH5α (ソ) | pSB1C3 | muta-CuMTS3 | EcoR1, Pst1 | Buffer H | |
| DH5α (F) | pSB1C3 | muta-CuMTS3 | EcoR1, Pst1 | Buffer H | |
| DH5α (N) | pSB1C3 | muta-CuMTS3 | EcoR1, Pst1 | Buffer H | |
| pSB1C3 | muta-CuMTS3 | EcoR1, Pst1 | Buffer H | ||
| pSB1C3 | Xba1 | FD buffer | |||
| DH5α (セ) | pSB1C3 | muta-CuMTS3 | Xba1 | FD buffer | |
| DH5α (ソ) | pSB1C3 | muta-CuMTS3 | Xba1 | FD buffer | |
| DH5α (F) | pSB1C3 | muta-CuMTS3 | Xba1 | FD buffer | |
| DH5α (N) | pSB1C3 | muta-CuMTS3 | Xba1 | FD buffer | |
AGE
| Lane | Sample | Enzyme | |||
|---|---|---|---|---|---|
| Marker | Host | Vector | Insert | ||
| 1 | pSB1C3 | ||||
| 2 | DH5α (セ) | pSB1C3 | muta-CuMTS3 | ||
| 3 | DH5α (ソ) | pSB1C3 | muta-CuMTS3 | ||
| 4 | DH5α (F) | pSB1C3 | muta-CuMTS3 | ||
| 5 | DH5α (N) | pSB1C3 | muta-CuMTS3 | ||
| 6 | 1kb Ladder | ||||
| 7 | DH5α (セ) | pSB1C3 | muta-CuMTS3 | EcoR1, Pst1 | |
| 8 | DH5α (ソ) | pSB1C3 | muta-CuMTS3 | EcoR1, Pst1 | |
| 9 | DH5α (F) | pSB1C3 | muta-CuMTS3 | EcoR1, Pst1 | |
| 10 | DH5α (N) | pSB1C3 | muta-CuMTS3 | EcoR1, Pst1 | |
| 11 | pSB1C3 | muta-CuMTS3 | EcoR1, Pst1 | ||
| 12 | 1kb Ladder | ||||
| 13 | pSB1C3 | Xba1 | |||
| 14 | DH5α (セ) | pSB1C3 | muta-CuMTS3 | Xba1 | |
| 15 | DH5α (ソ) | pSB1C3 | muta-CuMTS3 | Xba1 | |
| 16 | DH5α (F) | pSB1C3 | muta-CuMTS3 | Xba1 | |
| 17 | DH5α (N) | pSB1C3 | muta-CuMTS3 | Xba1 | |

Main Culture
| Sample | |||
|---|---|---|---|
| Host | Vector | Insert | Primer |
| DH5α(F) | pSB1C3 | muta-CuMTS3 | ---------- |
| DH5α(F) | pSB1C3 | muta-CuMTS3 | ---------- |
| DH5α(F) | pSB1C3 | muta-CuMTS3 | ---------- |
Cultivate these samples in 3mL of LB medium with ChlPhe at 30°C overnight.
September 21, 2014
KOBAYASHIMiniprep
| Sample | |||
|---|---|---|---|
| Host | Vector | Insert | Primer |
| DH5α(F) | pSB1C3 | muta-CuMTS3 | |
| DH5α(F) | pSB1C3 | muta-CuMTS3 | |
| DH5α(F) | pSB1C3 | muta-CuMTS3 | |
Restriction Digest
| Sample | Enzyme | Buffer | ||||
|---|---|---|---|---|---|---|
| Marker | Host | Vector | Insert | Primer | ||
| 1kb Ladder | ||||||
| pSB1C3 | BBa_J04450 | EcoR1 | Buffer H | |||
| pSB1C3 | BBa_J04450 | Pst1 | Buffer H | |||
| pSB1C3 | BBa_J04450 | EcoR1, Pst1 | Buffer H | |||
| pSB1C3 | BBa_J04450 | Spe1 | FD buffer | |||
| pSB1C3 | BBa_J04450 | Xba1 | FD buffer | |||
| pSB1C3 | BBa_J04450 | Spe1, Xba1 | FD buffer | |||
| pSB1C3 | BBa_J04450 | |||||
| λDNA-HindⅢ | ||||||
| F | pSB1C3 | muta-CuMTS3 | EcoR1 | Buffer H | ||
| F | pSB1C3 | muta-CuMTS3 | Pst1 | Buffer H | ||
| F | pSB1C3 | muta-CuMTS3 | EcoR1, Pst1 | Buffer H | ||
| F | pSB1C3 | muta-CuMTS3 | Spe1 | FD buffer | ||
| F | pSB1C3 | muta-CuMTS3 | Xba1 | FD buffer | ||
| F | pSB1C3 | muta-CuMTS3 | Spe1, Xba1 | FD buffer | ||
| F | pSB1C3 | muta-CuMTS3 | ||||
AGE
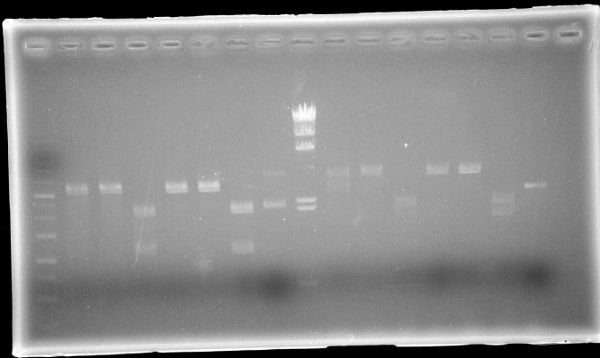
| Lane | Sample | Enzyme | ||||
|---|---|---|---|---|---|---|
| Marker | Host | Vector | Insert | Primer | ||
| 1 | 1kb Ladder | |||||
| 2 | pSB1C3 | BBa_J04450 | EcoR1 | |||
| 3 | pSB1C3 | BBa_J04450 | Pst1 | |||
| 4 | pSB1C3 | BBa_J04450 | EcoR1, Pst1 | |||
| 5 | pSB1C3 | BBa_J04450 | Spe1 | |||
| 6 | pSB1C3 | BBa_J04450 | Xba1 | |||
| 7 | pSB1C3 | BBa_J04450 | Spe1, Xba1 | |||
| 8 | pSB1C3 | BBa_J04450 | ||||
| 9 | λDNA-HindⅢ | |||||
| 10 | F | pSB1C3 | muta-CuMTS3 | EcoR1 | ||
| 11 | F | pSB1C3 | muta-CuMTS3 | Pst1 | ||
| 12 | F | pSB1C3 | muta-CuMTS3 | EcoR1, Pst1 | ||
| 13 | F | pSB1C3 | muta-CuMTS3 | Spe1 | ||
| 14 | F | pSB1C3 | muta-CuMTS3 | Xba1 | ||
| 15 | F | pSB1C3 | muta-CuMTS3 | Spe1, Xba1 | ||
| 16 | F | pSB1C3 | muta-CuMTS3 | |||
October
October 1, 2014
KOBAYASHIProtein Extraction (S. cerevisiae)
| Sample | |||
|---|---|---|---|
| Host | Vector | Insert | Primer |
| BY4247 | p427-TEF | ||
| BY4247 | p427-TEF | CuMTSE2 | pGEX6P-2F1, pGEX6P-2R |
| BY4247 | p427-TEF | CuMTS3 | pGEX6P-2F2, pGEX6P-2R |
| BY4247 | p427-TEF | CuMTS62 | pGEX6P-2F2, pGEX6P-2R |
SDS-PAGE
| Lane | Sample | |||
|---|---|---|---|---|
| Host | Vector | Insert | Primer | |
| 1 | BY4247 | p427-TEF | ||
| 2 | BY4247 | p427-TEF | CuMTSE2 | pGEX6P-2F1, pGEX6P-2R |
| 3 | BY4247 | p427-TEF | CuMTS3 | pGEX6P-2F2, pGEX6P-2R |
| 4 | BY4247 | p427-TEF | CuMTS62 | pGEX6P-2F2, pGEX6P-2R |
October 2, 2014
KOBAYASHIPreculture
| Sample | |||
|---|---|---|---|
| Host | Vector | Insert | Primer |
| BY4247 | p427-TEF | ||
| BY4247 | p427-TEF | CuMTS3 | pGEX6P-2F2, pGEX6P-2R |
Cultivate these samples in 3mL of SD medium with G418 at 30°C overnight.
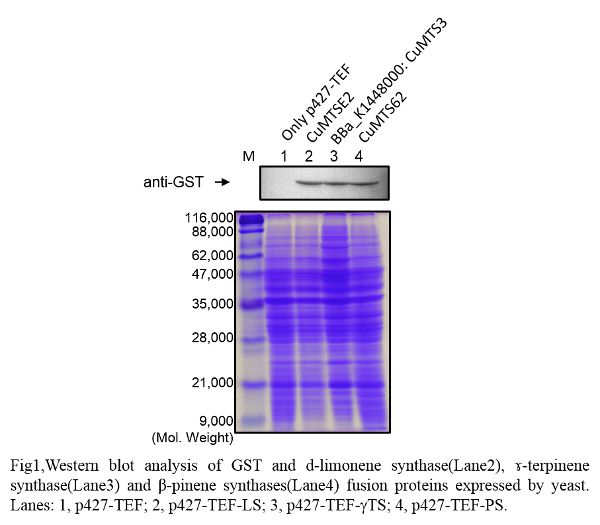
Western blot
| Lane | Sample | |||
|---|---|---|---|---|
| Host | Vector | Insert | Primer | |
| 1 | BY4247 | p427-TEF | ||
| 2 | BY4247 | p427-TEF | CuMTSE2 | pGEX6P-2F1, pGEX6P-2R |
| 3 | BY4247 | p427-TEF | CuMTS3 | pGEX6P-2F2, pGEX6P-2R |
| 4 | BY4247 | p427-TEF | CuMTS62 | pGEX6P-2F2, pGEX6P-2R |
October 3, 2014
KOBAYASHIMain culture
Cultivate following samples in Big Scale with G418 at 28°C overnight in shaken culture.
| Sample | |||
|---|---|---|---|
| Host | Vector | Insert | Primer |
| BY4247 | p427-TEF | ||
| BY4247 | p427-TEF | CuMTS3 | pGEX6P-2F2, pGEX6P-2R |
Restriction Digest
| Sample | Enzyme | Buffer | ||
|---|---|---|---|---|
| Vector | Insert | Primer | ||
| pSB1C3 | EcoR1, Spe1 | Buffer H | ||
| pSB1C3 | Xba1, Pst1 | Buffer H | ||
| Linearized pSB1A3 | EcoR1, Pst1 | Buffer H | ||
AGE
| Lane | Marker | Sample | Enzyme | ||
|---|---|---|---|---|---|
| Vector | Insert | Primer | |||
| 1 | 1kb Ladder | ||||
| 2 | pSB1C3 | EcoR1, Spe1 | |||
| 3 | pSB1C3 | Xba1, Pst1 | |||
| 4 | Linearized pSB1A3 | EcoR1, Pst1 | |||
DNA Refinement 1
| Sample | Enzyme | ||
|---|---|---|---|
| Vector | Insert | Primer | |
| pSB1C3 | EcoR1, Spe1 | ||
| pSB1C3 | Xba1, Pst1 | ||
| pSB1C3 | Xba1, Pst1 | ||
| Linearized pSB1A3 | EcoR1, Pst1, Dpn1 | ||
Ligation
| Vector | Sample | Enzyme | |
|---|---|---|---|
| Insert1 | Insert2 | ||
| pSB1A3 | EcoR1, Xba1, Spe1, Pst1 | ||
| pSB1A3 | EcoR1, Xba1, Spe1, Pst1 | ||
DNA ligase: DNA Ligation Kit ‹Mighty Mix› (TAKARA BIO INC.)
Transformation
| Host | Sample | ||
|---|---|---|---|
| Vector | Insert1 | Insert2 | |
| DH5α | pSB1A3 | ||
| DH5α | pSB1A3 | ||
October 4, 2014
KOBAYASHIColony Isolation
Isolate each colony from the plates which were inoculated overnight.
| Host | Sample | Section | ||
|---|---|---|---|---|
| Vector | Insert | Primer | ||
| DH5α | pSB1A3 | 1-16 | ||
| DH5α | pSB1A3 | a-p | ||
Monoterpene Extraction
| Host | Sample | ||
|---|---|---|---|
| Vector | Insert | Primer | |
| BY4247 | p427-TEF | ||
| BY4247 | p427-TEF | CuMTS3 | pGEX6P-2F2, pGEX6P-2R |
Samples were stocked in -4℃.
Preculture
| Host | Sample | ||
|---|---|---|---|
| Vector | Insert | Primer | |
| BY4247 | p427-TEF | CuMTSE2 | pGEX6P-2F2, pGEX6P-2R |
| BY4247 | p427-TEF | CuMTS62 | pGEX6P-2F2, pGEX6P-2R |
Cultivate samples in 3mL of SD medium with G418 at 30°C overnight.
October 5, 2014
KOBAYASHIMiniprep
| Host | Sample | |
|---|---|---|
| Vector | Insert | |
| DH5α | pSB1A3 | |
| DH5α | pSB1A3 | |
Restriction Digest and AGE
| Host | Sample | Enzyme | |
|---|---|---|---|
| Vector | Insert | ||
| DH5α | pSB1C3 | EcoR1, Pst1 | |
| DH5α | pSB1C3 | EcoR1, Pst1 | |
| pSB1C3 | K1448000 | EcoR1, Pst1 | |
October 8, 2014
KOBAYASHIGC-MS analysis
| Host | Sample | ||
|---|---|---|---|
| Vector | Insert | Primer | |
| BY4247 | p427-TEF | ||
| BY4247 | p427-TEF | CuMTS3 | pGEX6P-2F2, pGEX6P-2R |
Preculture
| Host | Sample | ||
|---|---|---|---|
| Vector | Insert | Primer | |
| BY4247 | p427-TEF | ||
| BY4247 | p427-TEF | CuMTS3 | pGEX6P-2F2, pGEX6P-2R |
October 9, 2014
KOBAYASHIMain Culture
| Host | Sample | ||
|---|---|---|---|
| Vector | Insert | Primer | |
| BY4247 | p427-TEF | ||
| BY4247 | p427-TEF | CuMTS3 | pGEX6P-2F2, pGEX6P-2R |
October 10, 2014
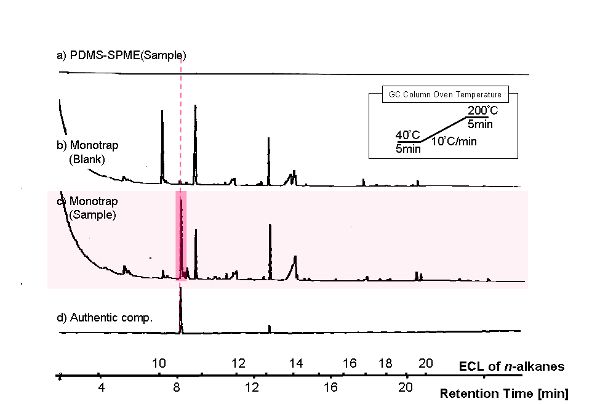
KOBAYASHIGC Analysis
| Host | Sample | ||
|---|---|---|---|
| Vector | Insert | Primer | |
| BY4247 | p427-TEF | ||
| BY4247 | p427-TEF | CuMTS3 | pGEX6P-2F2, pGEX6P-2R |
 "
"
