Team:Heidelberg/Parts
From 2014.igem.org
m |
m |
||
| Line 16: | Line 16: | ||
red-logo=true | red-logo=true | ||
| | | | ||
| - | subtitle=Discover our | + | subtitle=Discover our workpieces and standards |
| | | | ||
abstract= | abstract= | ||
Revision as of 21:31, 17 October 2014
– Discover our workpieces and standards
Our favorite Parts
The intein toolbox built by the iGEM Team Heidelberg includes three basic mechanisms. With our favorite parts we want to introduce you to the constructs enabling these priciples!
Sample Data Page
Take a look at our favorite Parts for circularization, fusion or tagging and see how our system works in the living cell. For that visit our Sample Data Page.Favorite Parts.
The iGEM Team Heidelberg 2014 had built a new biological system for the iGEM community integrating split-inteins. Intein splicing is a natural process that excises one part of a protein and leaves the remaining parts irreversibly attached. This great function allows you to modify your protein in numerous ways.
Creating a toolbox including all great functions and possibilities of inteins, we need a new standard for the scientific world of iGEM. This standard, the RFC of the iGEM Team Heidelberg 2014, allows us to easily and modulary work with split inteins.
Our favorite Parts represent the basic constructs of our toolbox – the Assembly and the Circularization construct, which are both tested in many methods and applications.
In the following we present you BBa_K1362000, the construct for circularization, BBa_K1362100 and BBa_K1362101, the N- and the C-construct for assembly. Take a look and visit the Partsregistry to read the associated documentation.
Circularization Construct. BBa_K1362000
BBa_K1362000
This part is an element of the Team Heidelberg 2014 intein toolbox and can be used as described in the new RFC[i]. The purpose of this tool is to provide an easy way to circularize any linear protein. While conserving the functionality of their linear counterpart, circular proteins can be superior in terms of thermostability, resistance against chemical denaturation and protection from exopeptidases. Moreover, a circular backbone can improve in vivo stability of therapeutical proteins and peptides. Using this part, Xylanase, lambda lysozyme and DNA methyltransferase (DNMT1) were successfully circularized.
Assembly Constructs. BBa_K1362100 and BBa_K1362101
BBa_K1362100
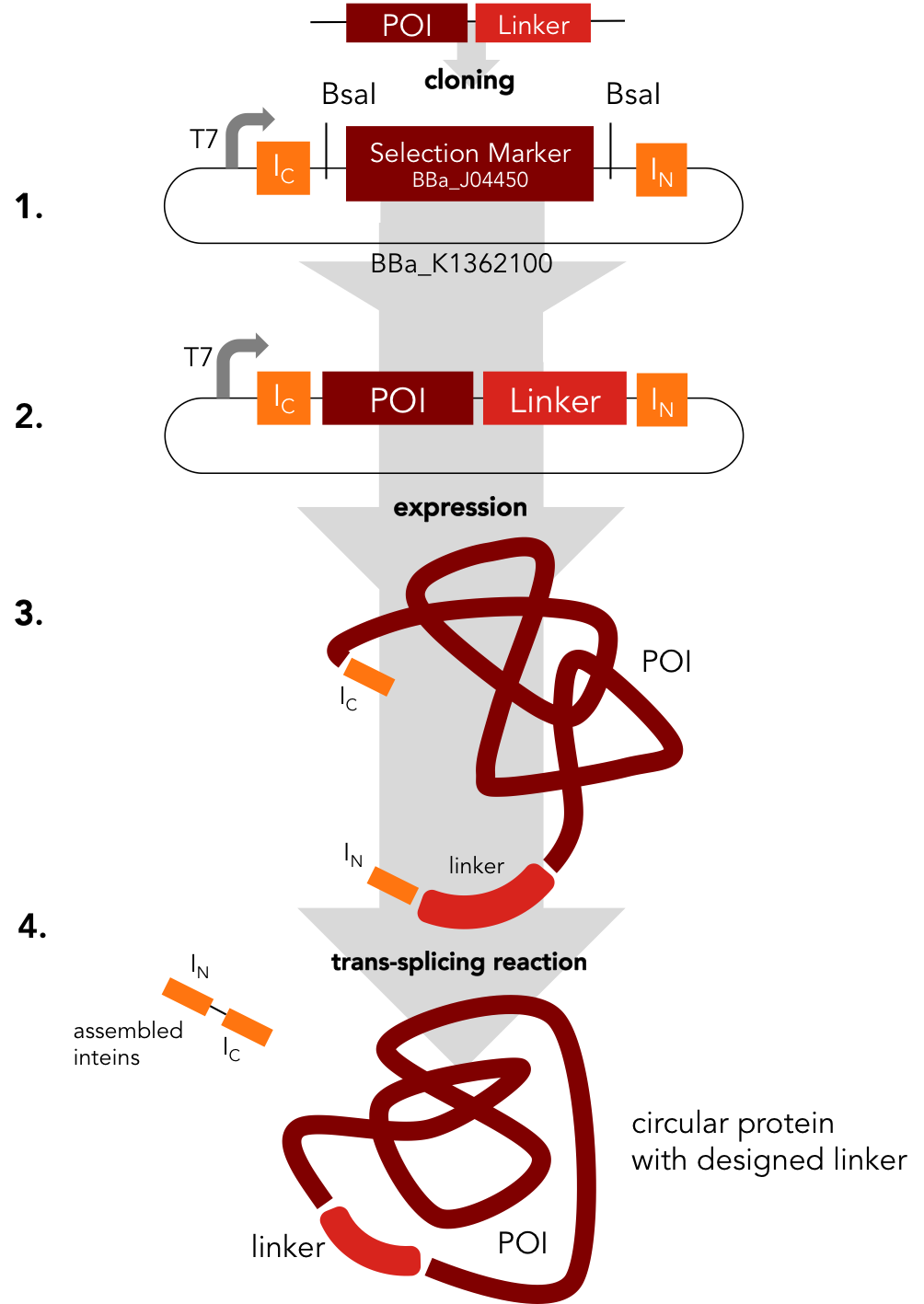
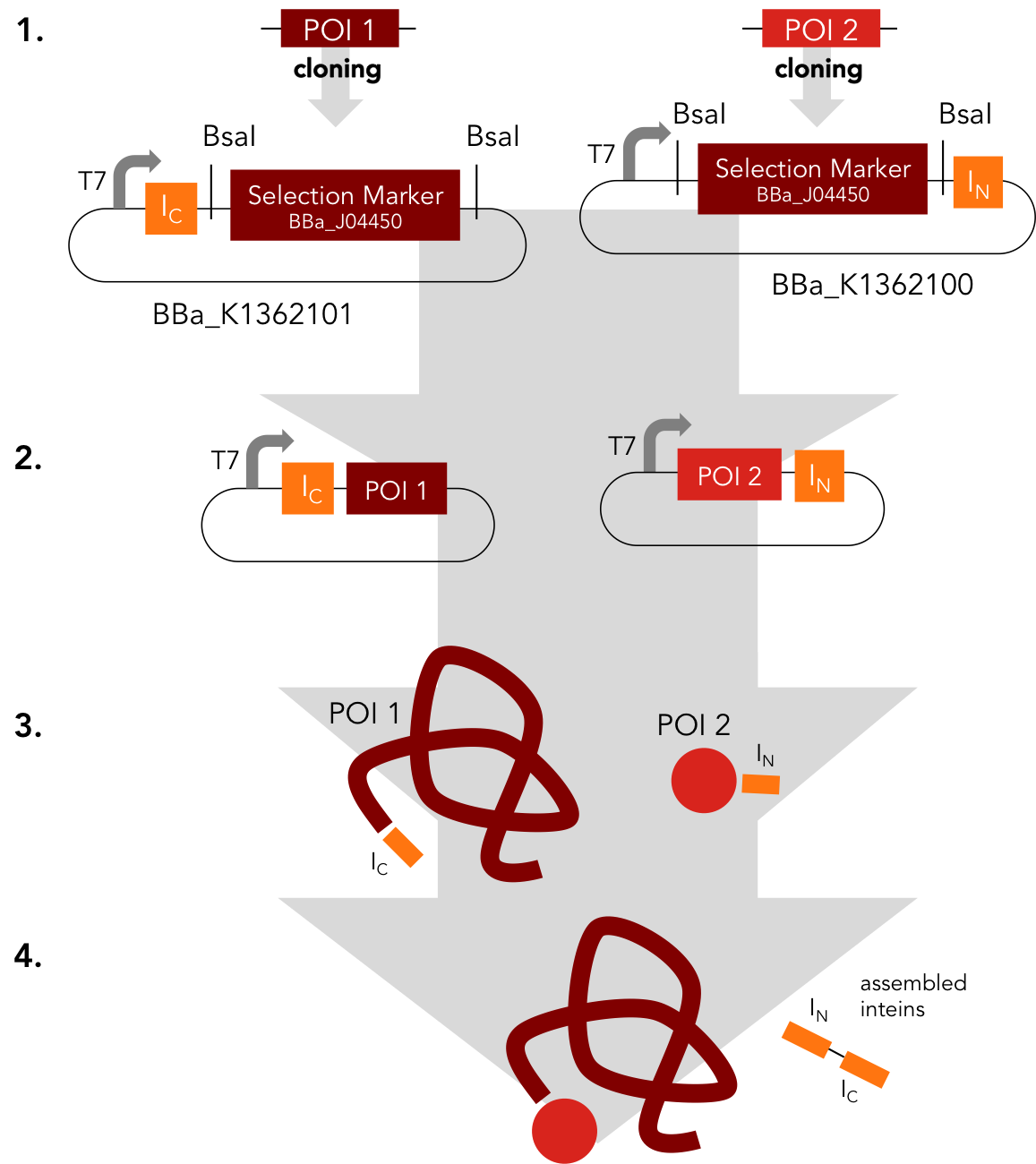
This intein assembly construct is part of our strategy for cloning with split inteins. Inteins are naturally occuring peptide sequences that splice out of a precursor protein and attach the remaining ends together to form a new protein. When splitting those intein sequence into an N-terminal and a C-terminal split intein one is left with a powerful tool to post-translationally modify whole proteins on the amino-acid sequence level. This construct was designed to express any protein of interest fused to the Nostoc punctiforme DnaE N-terminal split intein.

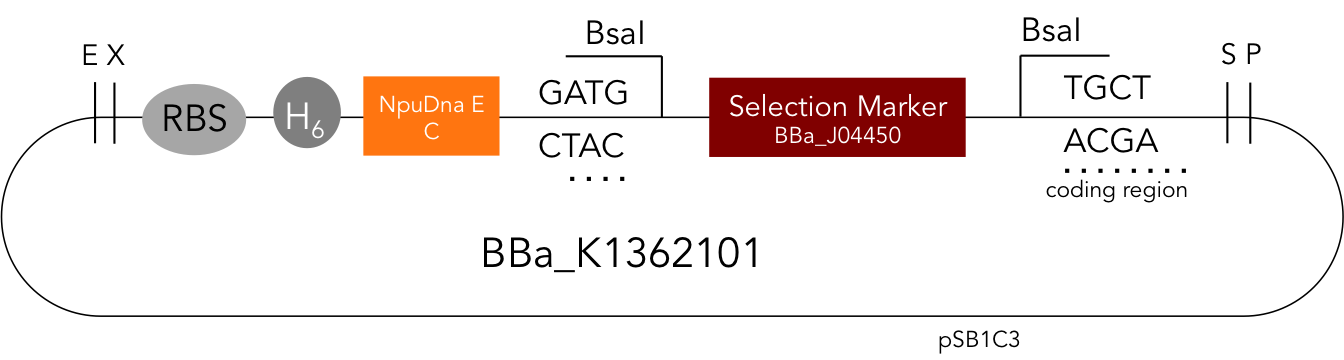
BBa_K1362101
BBa_K1362101 is the corresponding C-terminal construct to BBa_K1362100. Upon coexpression or mixture of the N- and C-constructs protein splicing takes place and the N- and C-terminal proteins of interest are irreversibly assembled via a newly formed peptide bond.This mechanism can be applied for a variety of different uses such as the activation of a protein through reconstitution of individually expressed split halves. See our split sfGFP experiment set a foundation that you guys can build on!

Sample Data Page for our favorite Parts.
Circularization Construct. BBa_K1362000
Assembly Construct. BBa_K1362100 and BBa_K1362101

Intein Library.
Inteins are the basic unity of our toolbox. They are integrated as extraneous polypeptide sequences into habitual proteins and do not follow the original protein function. Inteins perform an autocatalytic splicing reaction, where they excite themselves out of the host protein while reconnecting the remaining chains on both end, so called N and C exteins, via a new peptide bond. Read more about it in our project background!
To characterize the different types and groups of split-inteins and inteins we collect many details about them to develop a intein library. It gives you a great and clear overview about the most important facts.
| Split intein | Special features | Size N [aa] | Size C [aa] | Reaction properties | Origin | References |
|---|---|---|---|---|---|---|
| Npu DnaE | fast; robust at high temperature range and high-yielding trans-splicing activity, well characterised requirements | 102 | 36 | t1/2 = 63s , 37°C , k=~1x10^-2 (s^-1); activity range 6 to 37°C | S1 natural split intein, Nostoc punctiforme | [1] [2] |
| Ssp DnaX | cross-reactivity with other N-inteins, transsplicing in vivo and in vitro, high yields | 127 | 6 | k=~1.7x10^-4(s^-1); efficiency 96% | engineered from Synechocystis species | [3] [4] |
| Ssp GyrB | very short Nint facilitates trans-splicing of synthetic peptides | 6 | 150 | k=~1x10^-4(s^-1), efficiency 40-80% | S11 split intein enginered from Synechocystis species, strain PCC6803 | [4] [5] |
| Ter DnaE3 | trans-splicing activity with high yields | 102 | 36 | k=~2x10^-4(s^-1), efficiency 87% | natural split intein, Trichodesmium erythraeum | [4] [6] |
| Ssp DnaB | relatively fast | t1/2=12min, 25°C, k=~1x10^-3(s^-1) | engineered from Synechocystis species, strain PCC6803 | [2] | ||
| Gp41-1 | fastes known reaction | 88 | 38 | t1/2=20-30s, 37°C, k=~1.8x10^-1 (s^-1); activity range 0 to 60°C | natural split intein, Cyanophage | [7] [8] |
References
[1] Iwai, H., Züger, S., Jin, J. & Tam, P.-H. Highly efficient protein trans-splicing by a naturally split DnaE intein from Nostoc punctiforme. FEBS Lett. 580, 1853–8 (2006).
[2] Zettler, J., Schütz, V. & Mootz, H. D. The naturally split Npu DnaE intein exhibits an extraordinarily high rate in the protein trans-splicing reaction. FEBS Lett. 583, 909–14 (2009).
[3] Song, H., Meng, Q. & Liu, X.-Q. Protein trans-splicing of an atypical split intein showing structural flexibility and cross-reactivity. PLoS One 7, e45355 (2012).
[4] Lin, Y. et al. Protein trans-splicing of multiple atypical split inteins engineered from natural inteins. PLoS One 8, e59516 (2013).
[5] Appleby, J. H., Zhou, K., Volkmann, G. & Liu, X.-Q. Novel Split Intein for trans-Splicing Synthetic Peptide onto C Terminus of Protein. J. Biol. Chem. 284, 6194–6199 (2009).
[6] Liu, X.-Q. & Yang, J. Split dnaE genes encoding multiple novel inteins in Trichodesmium erythraeum. J. Biol. Chem. 278, 26315–8 (2003).
[7] Carvajal-Vallejos, P., Pallissé, R., Mootz, H. D. & Schmidt, S. R. Unprecedented rates and efficiencies revealed for new natural split inteins from metagenomic sources. J. Biol. Chem. 287, 28686–96 (2012).
[8] Dassa, B., London, N., Stoddard, B. L., Schueler-Furman, O. & Pietrokovski, S. Fractured genes: a novel genomic arrangement involving new split inteins and a new homing endonuclease family. Nucleic Acids Res. 37, 2560–73 (2009).

Our Backbones.
Standard BioBrick cloning is a universal way of putting two BioBrick parts together to build a new BioBrick part. Despite several alternative cloning methods allow the assembly of multiple parts at one its simplicity and the broad availability of compatible parts keep it the 'de facto' standard of the iGEM-community.
Using standard BioBrick cloning, the generation of translationally active parts requires often more than one round of cloning. The ability to easily test the functionality of a protein before cloning them into complicated circuits has the potential to prevent many unsuccessful experiments of iGEM teams and may improve the characterization of the parts in the parts registry. However the extra amount of work required to clone such an additional construct may inhibit this behavior. We therefore improved the standard plasmids pSB1X3 and pSB4X5 by inserting a lacI repressible T7 promoter directly upstream to the BioBrick prefix of those plasmids. This promoter is completely inactive in 'E. coli' strains lacking a T7 RNA polymerase such as TOP10 or DH10beta bute inducible in strains carrying the T7 RNA polymerase under a lacI repressible promoter such as DE3 strains. This enables the use of the same backbone for cloning and over expression. Using 3A assembly a translational active part can be cloned from an RBS and a coding part in one step while maintaining the full flexibility of standard BioBrick assembly. These new RFC 10 conform backbones eliminate one cloning step needed for the expression and thus the characterization of a newly BioBricked protein. Version number 30 was claimed for the high copy variants and version number 50 for the low copy variants.
High copy BioBrick expression backbone:
- pSB1A30(Part:BBa_K1362091): High copy BioBrick cloning/expression backbone carrying Amp resistance
- pSB1C30(Part:BBa_K1362092): High copy BioBrick cloning/expression backbone carrying Cm resistance
- pSB1K30(Part:BBa_K1362093): High copy BioBrick cloning/expression backbone carrying Kan resistance
- pSB1T30(Part:BBa_K1362094): High copy BioBrick cloning/expression backbone carrying Tet resistance
Low copy BioBrick expression backbone:
- pSB4A50(Part:BBa_K1362095): High copy BioBrick cloning/expression backbone carrying Amp resistance
- pSB4C50(Part:BBa_K1362096): High copy BioBrick cloning/expression backbone carrying Cm resistance
- pSB4K50(Part:BBa_K1362097): High copy BioBrick cloning/expression backbone carrying Kan resistance
Because of the great experience we had using our expression vectors, we sent them to the iGEM team Aachen and Tuebingen. We helped them solving their problems with the expression of their products.

List of Parts

 "
"


