Team:Heidelberg/Project
From 2014.igem.org
| Line 336: | Line 336: | ||
<div id="abstract-dropdown" class="row abstract-special dark-grey"> | <div id="abstract-dropdown" class="row abstract-special dark-grey"> | ||
<div class="col-lg-offset-1 col-lg-2"> | <div class="col-lg-offset-1 col-lg-2"> | ||
| - | <h4><img id="dropdownImg" src="/wiki/images/7/70/Heidelberg_Abstract-dropdown.png"/> Abstract</h4> | + | <h4><img id="dropdownImg" src="/wiki/images/7/70/Heidelberg_Abstract-dropdown.png"/> Abstract</h4> <p>Proteins are the functional basis of all biological porcesses ...</p> |
</div> | </div> | ||
<!--<div class="col-lg-9"> | <!--<div class="col-lg-9"> | ||
<span>Click to read full abstract</span> | <span>Click to read full abstract</span> | ||
</div>--> | </div>--> | ||
| - | <div id="abstract-content" class="col-lg-12 | + | <div id="abstract-content" class="col-lg-12" > |
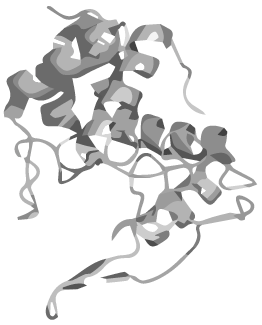
Proteins are the functional basis of all biological processes and being able to control and improve their functions through design and engineering is one of the fundamental goals of synthetic biology. While conventional proteins subsist as chains of amino acids with defined beginning and end, nature has found a curious way of enhancing a protein capabilities: circularization. | Proteins are the functional basis of all biological processes and being able to control and improve their functions through design and engineering is one of the fundamental goals of synthetic biology. While conventional proteins subsist as chains of amino acids with defined beginning and end, nature has found a curious way of enhancing a protein capabilities: circularization. | ||
In head to tail circularized peptides the terminal amino acids are joined together just like in the rest of the chain, forming a circular structure. Such peptides have been discovered in all kingdoms of life during the past years and they are unified by an extreme stability towards high temperatures, proteases and changes in pH.</p> | In head to tail circularized peptides the terminal amino acids are joined together just like in the rest of the chain, forming a circular structure. Such peptides have been discovered in all kingdoms of life during the past years and they are unified by an extreme stability towards high temperatures, proteases and changes in pH.</p> | ||
Revision as of 15:57, 17 October 2014
 Abstract
Abstract
Proteins are the functional basis of all biological porcesses ...
Given these attractive features of circular proteins, methods to circularize otherwise linear proteins have been devised, one of which is based on autocatalytic protein domains called inteins. We have applied the principle of circular peptides to synthetic biology by introducing a BioBrick-based, standardised method for circularizing any protein using inteins.
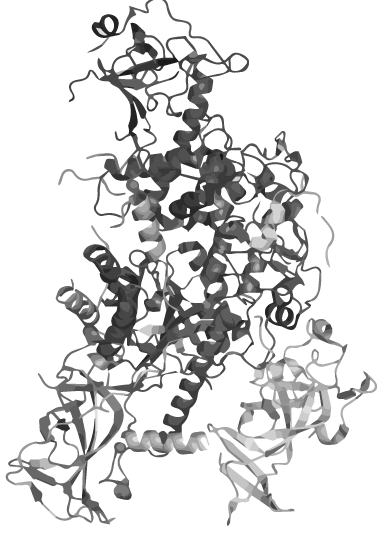
Synthetically connecting a protein's termini without disrupting its 3D structure and function is, however, a delicate task which has so far been accomplished only for relatively small proteins whose ends lie close to each other. We therefore saw the need for a comprehensive software that predicts the process of circularization. With CRAUT we have brought into existence a powerful open-source software to predict an optimal linker to support circularization of a protein preserving its 3D structure and function.
Due to our lack of calculating power we deployed this software on the distributed computing platform BOINC in an initiative we call iGEM@home.
Based on our software predictions, we constructed linkers to circularize the 871 a.a. long methyltransferase Dnmt1 and provide data suggesting that circular DNMT1 is more functional than its linear counterpart at high temperatures. Our results have strong implications for developing an innovative PCR-based technique that could revolutionize epigenetic studies and cancer research by maintaining the methylation pattern of the DNA template during amplification.
Eventually, inteins can be used to post-translationally modify any protein in a multitude of ways going far beyond circularization. We therefore created a BioBrick-based intein toolbox to allow for easy and standardised protein manipulation. We think that our toolbox will be invaluable to many systems biology projects aimed at dissecting or re-engineering the function of cellular networks.
 "
"