Team:Oxford/codon optimisation
From 2014.igem.org
(Difference between revisions)
| (7 intermediate revisions not shown) | |||
| Line 24: | Line 24: | ||
</div> | </div> | ||
<div style="background-color:white; border-bottom-left-radius:10px;border-radius:10px; padding-left:10px;padding-right:10px;min-width:300px;margin-top:-50px;"> | <div style="background-color:white; border-bottom-left-radius:10px;border-radius:10px; padding-left:10px;padding-right:10px;min-width:300px;margin-top:-50px;"> | ||
| - | <a href=" | + | <a href="https://static.igem.org/mediawiki/2014/3/3d/OxigemLAB_BOOK.pdf" target="_blank"><img src="https://static.igem.org/mediawiki/2014/5/50/OxigemLabbook.png" style="position:absolute;width:6%;margin-left:84%;margin-top:-13%;z-index:10;"></a> |
<a href="https://static.igem.org/mediawiki/2014/1/16/Oxigem_LAB_PROTOCOLS.pdf" target="_blank"><img src="https://static.igem.org/mediawiki/2014/a/a4/OxigemProtocols.png" style="position:absolute;width:6%;margin-left:91%;margin-top:-13%;z-index:10;"></a> | <a href="https://static.igem.org/mediawiki/2014/1/16/Oxigem_LAB_PROTOCOLS.pdf" target="_blank"><img src="https://static.igem.org/mediawiki/2014/a/a4/OxigemProtocols.png" style="position:absolute;width:6%;margin-left:91%;margin-top:-13%;z-index:10;"></a> | ||
<br> | <br> | ||
| - | <h1>Introduction: | + | <h1>Introduction: Codon Optimisation</h1> |
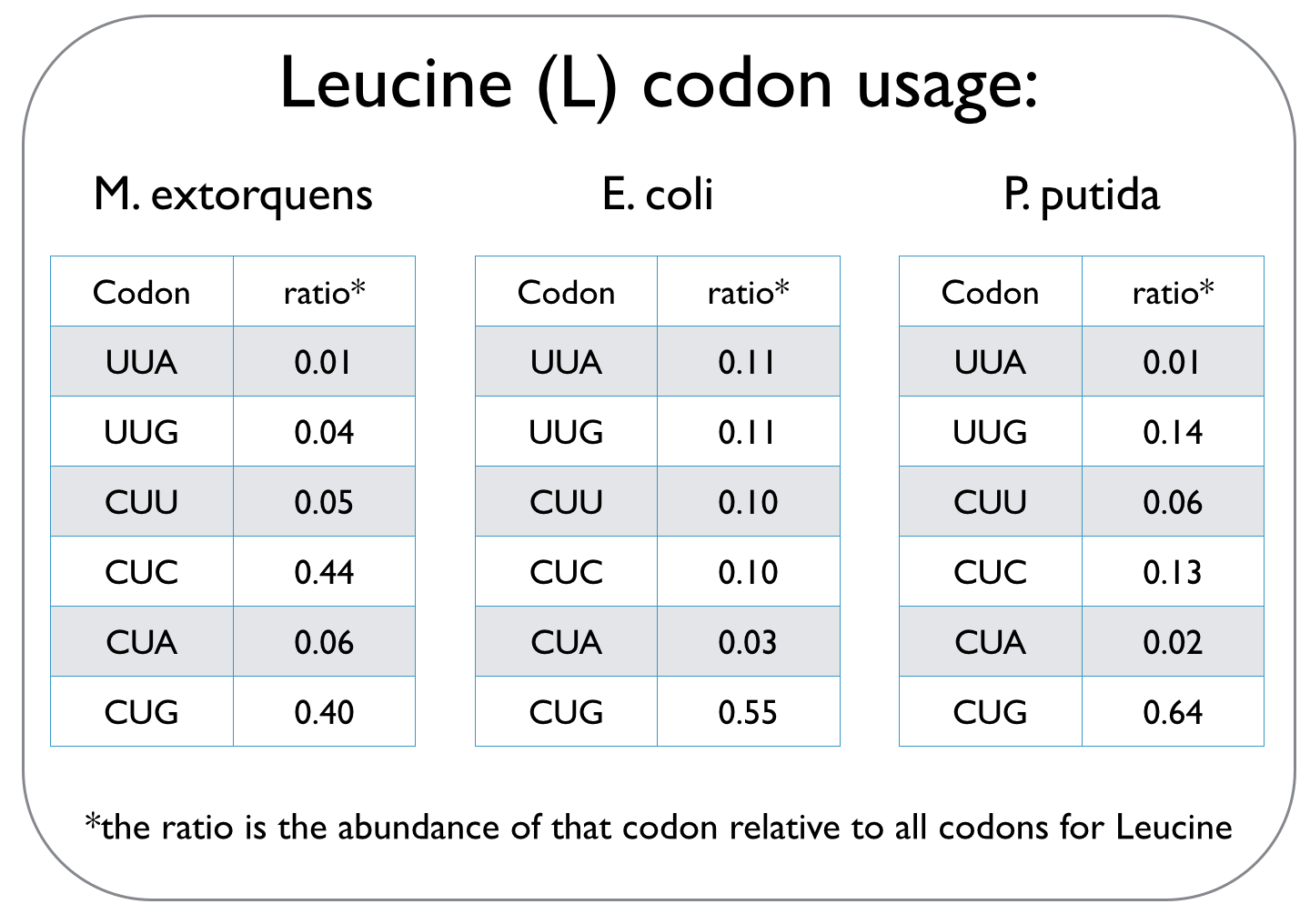
In our quest to optimise the process of DCM breakdown, the first step was to move the genes we were interested in into well-characterised host strains. The source bacterium Methylobacterium extorquens DM4 proved very difficult to grow in the lab, and took its leisurely time once it did decide to grow. It's also not a particularly well-studied bacterium, unlike E. coli and P. putida, which we decided to work with. This section explains the concept of codon optimisation, why we used it, and how we did so. <br><br> | In our quest to optimise the process of DCM breakdown, the first step was to move the genes we were interested in into well-characterised host strains. The source bacterium Methylobacterium extorquens DM4 proved very difficult to grow in the lab, and took its leisurely time once it did decide to grow. It's also not a particularly well-studied bacterium, unlike E. coli and P. putida, which we decided to work with. This section explains the concept of codon optimisation, why we used it, and how we did so. <br><br> | ||
</div> | </div> | ||
| Line 42: | Line 42: | ||
</div> | </div> | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | <div class="row"> | ||
| + | <a href="#show14" class="show wetlab-row" id="show14"><div class="wetlab"> | ||
| + | <h1white>Expression of dcmA in E.coli</h1white> | ||
| + | <img src="https://static.igem.org/mediawiki/2014/4/4d/Oxford_plus-sign-clip-art.png" style="float:right;position:relative; width:2%;" /> | ||
| + | </div></a> | ||
| + | |||
| + | <a href="#hide14" class="hide" id="hide14"><div class="wetlab"> | ||
| + | <h1white>Expression of dcmA in E.coli</h1white></div></a> | ||
| + | <div class="list"> | ||
| + | <div class="white_news_block2"> | ||
| + | Using the pRSFDuet vector and codon-optimised dcmA as the insert, we expressed dcmA fused to sfGFP in E.coli. The fused protein has a total molecular weight of 61.84 kDa. We managed to show this using western blots as indicated in part A of the figure below: | ||
| + | |||
| + | <img src="https://static.igem.org/mediawiki/2014/e/e5/DCMAsfGFP.jpg" style="float:left;position:relative; width:60%;margin-left:20%;margin-right:20%" /> | ||
| + | |||
| + | <br> | ||
| + | |||
| + | As shown in this figure, we have induced expression of dcmA-sfGFP with 0, 0.1 and 1 mM concentrations of IPTG (the inserts in pRSFDuet are under lac-control). Part B of this figure indicates OD and fluorescence readings of the three samples, showing how sfGPF (and thus expression levels of dcmA) increase at higher IPTG concentrations. | ||
| + | |||
| + | <br> | ||
| + | |||
| + | |||
| + | |||
| + | </div> | ||
| + | |||
| + | </div> | ||
| + | </div> | ||
Latest revision as of 02:18, 18 October 2014
 "
"