Team:Evry/Notebook
From 2014.igem.org
| Line 195: | Line 195: | ||
<h5>Sensing</h5> | <h5>Sensing</h5> | ||
{{:Team:Evry/Notebook/Sensing}} | {{:Team:Evry/Notebook/Sensing}} | ||
| + | <h5>Interlab study</h5> | ||
| + | {{:Team:Evry/Notebook/Interlabstudy}} | ||
<html> | <html> | ||
Revision as of 13:45, 14 August 2014

Notebook
RNAseq
Week 1
Here
Transformation
Week 1
Week 1
HERE HERE
Sensing
Notebook - Sensing

Mutation of the Pst1 site
Digestion of plasmid containing DmpR + GFP on three strains of E.coli (DH5a, Top10 and Bl21) by EcoR1 (wells 1), Pst1 (wells 2) and EcoR1+Pst1 (wells 3).

We have the good construction in DH5a AND tOP 10 STRAINS.

Assembling of the parts
Ligation of all parts
Transformation on E.coli
Purification of palsmids with NucleoSpin Plamsid Kit (Macherey Nagel) on 4 colonies
Digestion with EcoRI

All colonies contain the construction of all parts.

Sensor construction bphR2/PbpR1
A new tentative of golden gate has been done with modification of volumes of each part:
- J23114-rbs : 0,12µL
- bphR2: 1,05µl
- bphR1:0,31µl
- RFP : 0,64µl
- Terminator: 0,67µl
- pSB1C3,G3: 1µl
The mix enzyme was inchanged and the program is of 8h
Sep 08

Sensor construction bphR2/PbpR1
Results PCR clean-up:
- J23114-rbs : 76ng/µl
- bphR2: 76,8 ng/µl
- bphR1:126,4 ng/µl
- RFP : 98,3ng/µl
- Terminator: 42,4ng/µl
- pSB1C3,G3: 1µl
Sep 07

Sensor construction bphR2/PbpR1
Miniprep was made for each part and the nanodrop gives:
- J23114-rbs: 205 ng/µl
- bphR2: 227,5ng/µl
- bphR1:57,2ng/µl
- RFP: 74,2ng/µl
- terminator:54,6ng/µl
- pSB1C3,G3: 182,7ng/µl
A new PCR has been done by using Q5 DNA polymerase for all the parts expected for the terminator (one taq polymerase)
Sep 05

Sensor construction bphR2/PbpR1
As the first golden gate test failed, we retry at the beginning. Parts were cultured in 10ml LB + 10µL Cam for bphR2, bphR1, RFP, Terminator, pSB1C3 and in 10ml LB + 10µL Amp for J23114 and stored at 37°C overnight
Sep 04

Sensor construction bphR2/PbpR1
There is not colonies from the transformation of golden gate product in pSB1C3,G3.
Sep 03

Sensor construction bphR2/PbpR1
Transformation has made with golden gate product and 5µL of the product migrated :

There is'nt DNA in the mix
Aug 29

Sensor construction bphR2/PbpR1
PCR colony has been done with colonies of the transformation bphR2 in pSB1C3. The tail expected was 1242bp and we obtained it:

A nanodrop has been done on the colony 1 (48ng/µl) and colony 2 (4&,4ng/µl) and the colony has been send to sequencing
With PCR clean up of each part, golden gate has been done. Volumes for each part has been calculated with cloning bench application.
Mix DNA contains:
- J23114-RBS (82,1ng/µl):0,06 µl
- bphR2 (139,3 ng/µl) : 0,327µl
- bphR1 (101,1ng/µl): 0,21µl
- RFP (75,3 ng/µl): 0,46µl
- Terminator (28,1ng/µl) : 0,37µl
- pSB1C3,G3 (107,2ng/µl) : 0,93µl
Mix enzyme contains:- 1,5µl 10X T4 ligase buffer
- 0,5µl BSAI
- 0,5µl T4 ligase
- 2,5µl
Aug 28

Sensor construction bphR2/PbpR1
Some colonies had grown after the transformation of bphR2 in pSB1C3:

Products PCR from 26th august has been migrated :

Expected for the terminator, we have bands at the good tail. So for the terminator, an other PCR has been made but by using the one taq polymerase instead of Q5 DNA polymerase and we obtained the good band:

Aug 27

Sensor construction bphR2/PbpR1
We wanted to assemble the construction by using golden gate. After to have received primers, PCR was done to amplify J23114-RBS, bphR2, bphR1-RBS, RFP and terminator with golden gate primers. The tail of each part is:
- J23114-RBS = 82bp
- bphR2 mutated = 929bp
- bphR1-RBS = 340bp
- RFP = 732bp
- Terminator = 162bp
Simultaneously, the product of digestion of bphR2 and pSB1C3 has been migrated to verify the digestion then, bphR2 and pSB1C3 has been ligated and we have done a heat shock transformation.
Aug 26

Sensor construction bphR2/PbphR1 :
Miniprep of pSB1C3 was done => 68,9ng/µl and the plasmid was digested with EcoRI-HF and pstI.
Digestion products of pSB1C3 and bphr2 has been migrated on gel 1X agarosis. Bands expected were:
- for pSB1C3: one band at 2069bp (vector) and one band at 1070bp (insert)
- for bphr2: one band at 946 pb (insert) and one band at 1300bp (vector)
We obtained the good bands so an extraction on gel was done to recover only pSB1C3 without its insert and only bphr2 gene. Aug 23

Transformation plate observation:
- BBa_E1010: 50-60 colonies
- BBa_J23114: 150-200 colonies
- BBa_B0015: 40-50 colonies
- PSB1C3G3: > 1000 red colonies
A PCR was performed on 8 colonies for BBa_J23115 (K823012) following the protocol Table 3 and 4.
Samples are loaded on a 1% agarose gel, 10 µL of sample + 2 µl of loading dye 6X per well. Gel ran 45 minutes at 100 mV.
Sensor construction bphR2/PbphR1 :
One colony of the transformation of pSB1C3 was incubated in 3ml LB + 3µL Cam, at 37°C, overnight

Sensor construction bphR2/PbpR1
BBa_J23114 was resuspended with 10 µL sterile water in order to obtain a DNA concentration around 0.2 ng/µl (according to the registry). Solution was transferred into one 1 ml eppendorf tube and stored at -20°C.
Transformations of constitutive promoter BBa_J23114, RFP BBa_E1010 and terminator BBa_B0015 and vector pSB1C3G3 was done on DH5alpha as followed:
- Remove E. coli competent tubes from -80°C and keep it on ice
- Add 3 µl of template (here solubilized plasmids from the registry distribution kit) and mix gently
- Incubate 10 minutes on ice
- Perform an heat shock 30 seconds at 42°C
- Incubate 2 minutes on ice
- Add 2 ml of LB medium and incubate 60 minutes at 37°C with an agitation at 200 rpm
- Plate 200 µl of pSB1C3G3, BBa_B0015 and E1010 on a chloramphenicol LB agar plate and ampicilline LB agar plate for BBa_J23114
- Incubate plate overnight at 37°C
We received primers.
pSB1C3 was digested with EcoRI and pstI and a ligation with bphR2 was done before the transformation in DH5a.
50 µL were plated on Cam Lb plate and incubated at 37°C overnight
Aug 21

Sensor construction bphR2/PbphR1
We received sequencing reads: that match perfectly to the registry sequence.
26DJ54
AATAGGCGTTATCACGAGGCAGAANTTCAGATAAAAAAAATCCTTAGCTTTCGCTAAGGATGATTTCTGGAATTCGCGGCCGCTTCTAGAGATGTCCCTGG
GTGACATGCGTGATTTGGCCGCCACGCGGATCGCGCTCGAGAGCGAAGCGTTACGCCAAAGCGTGCTGAATGGTGACGCTGAATGGGAGGCGCGGATCGTCAGTTC
GTTTCACCGACTGTCATTGATTGAAGAGCCCACGATGCGGGATCCGGCTCGCTGGTTTAATGAGTGGGAGCCAGTCAACCGCGGTTTTCACGAAGCTCTTATCTCT
GCCTGTTCGTCCGTCTGGATCCGGCGGTTCCTGTCCATCCTGTATGTGCATATGGAGCGCTACCGCCGATTGACTGCTTACTAGTAGCGGCCGCTGCAGTCCGGCA
AAAAAGGGCAAGGTGTCACCACCCTGCCCTTTTTCTTTAAAACCGAAAAGATTACTTCGCGTTATGCAGGCTTCCTCGCTCACTGACTCGCTGCGCTCGGTCGTTC
GGCTGCGGCGAGCGGTATCA
GCTCACTCAGGG
26DJ55
CGAGTCAGTGAGCGAGGAAGCCTGCATAACGCGAAGTAATCTTTTCGGTTTTAAAGAAAAAGGGCAGGGTGGTGACACCTTGCCCTTTTTTGCCGGACTGC
AGCGGCCGCTACTAGTAAGCAGTCAATCGGCGGTAGCGCTCCATATGCACATACAGGATGGACAGGAACCGCCGGATCCAGACGGACGAACAGGCAGAGATAAGAG
CTTCGTGAAAACCGCGGTTGACTGGCTCCCACTCATTAAACCAGCGAGCCGGATCCCGCATCGTGGGCTCTTCAATCAATGACAGTCGGTGAAACGAACTGACGAT
CCGCGCCTCCCATTCAGCGTCACCATTCAGCACGCTTTGGCGTAACGCTTCGCTCTCGAGCGCGATCCGCGTGGCGGCCAAATCACGCATGTCACCCAGGGACATC
TCTAGAAGCGGCCGCGAATTCCAGAAATCATCCTTAGCGAAAGCTAAGGATTTTTTTTATCTGAAATTCTGCCTCGTGATACGCCTATTTTTATAGGTTAATGTCA
TGATAATAATGGTTTCTTAGA
Plasmid with bphR2 gene was purified with NucleoSpin Plamsid protocol (Macherey-Nagel) => we obtained 227,5ng/µL
This plasmid was digested according this protocol:
- Add:
- sterilized water: qsp 20µL
- template DNA : 500ng
- buffer 2.1: 2µL
- BSA: 0,2µL
- EcoRI: 1µL
- PstI: 1µL
- Reverse for mix
- Incubate at 37°C during 45mn
- Incubate at 80°C during 20mn
- Ligation was not possible because pSB1C3 plasmid was not digested so the digested plasmid (bphR2 gene) was stored at -20°C
Survival test on E.coli BL21:
Bacteria survive again for different concentrations but they were very concentrated
A dilution of the medium has been done for the positive control and the six different concentrations:


PCR using VF2 and VR primer
Q5 polymerase
Expected bands :
DmpR: 2038 bp
GFP B0031: 1331 bp
GFP B0032: 1330 bp
Digestion
From newly extracted DNA
DmpR: SpeI&PstI
GFP B0031/32: XbaI&PstI

VF2/VR PCR products are still in agreement with the expected size either for DmpR and GFP B0031/32.
GFP 31/32 digestion products were in agreement with the expected size.
DmpR digestion by SP is still displaying 2 close bands profile.
Aug 20

Sensor construction bphR2/PbphR1:
A colony after the DH5a transformation was cultured in 3mL of LB + 3µL Amp, at 37°C, 200rpm, overnight
Survival test on E.coli BL21:
Bacteria continue to grow for all concentrations of 2 hydroxy-3',4'dichlorobiphenyl.

Dna extraction
Machery Nagel DNA purification Kit (PROTOCOL)
PCR using VF2 and VR primer
Q5 polymerase
Expected bands :
DmpR: 2038 bp
GFP B0031: 1331 bp
GFP B0032: 1330 bp
Digestion
DmpR: SpeI&PstI
GFP B0031/32: XbaI&PstI
Analysis
VF2/VR PCR products were in agreement with the expected size either for DmpR and GFP B0031/32.
GFP 31/32 digestion products were in agreement with the expected size.
However DmpR digestion revealed an unexpected profile.
Gel extraction
Extraction of GFP B0031/32 digestion product [XbaI-PstI]
After confirmation of relevant electrophoresis bands, cut the gel all around the band and as close as possible to it.
Place the resulting piece into a 2ml microcentrifuge tube. Add 1µl of Binding buffer to 1 µg of gel. Place the tube at 55°C to 60°C during 10min or until gel turn completely liquid.
Follow classical DNA purification protocol.
Machery Nagel kit (PROTOCOL 2)

Sensor construction bphR2/PbphR1:
bphR2 gene, synthetized by Eurofins in a plasmid, was dissolved in 23,5µL of elution buffer because it was lyophilized.
Concentration obtained = 200ng/µL
DH5a chimiocompetents were transformed with this plasmid according to this protocol:
- Remove DH5a from -80°C (about 200µL) and let them on the ice
- Add 100ng of plasmid at DH5a and let during 30mn on ice
- Make a heat shock by putting bacteria at 42°C during 30s-1mn
- Let 1h at 37°C
- Plate 100µL of the culture on LB-agar-Amp
- Incubate at 37°C overnight, 200rpm

Aug 17


Cell culture of E.coli received from registry
BBa_K1031211 (Pr-DmpR )
BBa_K1031221 (P0-RBS B0031-GFP)
BBa_k1031222 (P0-RBS B0032-GFP)
Liquid : 3ml of Luria Broth 1x + 34µg/L Chloramphenicol in a 15ml falcon at 37°C shaking
On plate : 25ml LB 1x + 25ml
Agarose 1x + 34µg/L Chloramphenicol
Observation: DmpR bacteria have difficulties to grow compared with GFP bacteria

Left : BBa_K1031221 (P0-RBS B0031-GFP)
Right : BBa_k1031222 (P0-RBS B0032-GFP)

Survival test on E.coli BL21:
- Serial dilution of compound was made from 10(-2) mol/L to 10(-8) mol/L

- 300µL of E.coli BL21 were added in each eppendorf tube.
We had 2 control tubes:- A negative control which contains 2,7mL of M9 medium + 300µL of compound
- A positive control which contains 2,7mL of M9 medium + 300µL of E.coli BL21
- Tubes were incubated at 37°C overnight, 200rpm Aug 15

Survival test on E.coli BL21:
This test allowed to know if E.coli can survive at different concentrations of 2 hydroxy-3',4'dichlorobiphenyl
- For that, BL21 culture was removed from the -80°C and culturing in 4 mL of LB
- E.coli was incubated overnight at 37°C, 200rpm

Survival test on E.coli BL21:
10mg of 2 hydroxy,3'-4'dichlorobiphenyl have been received. The compound was lyophilized so it's has been dissolved in 17mL of DMSO in order to have a final concentration of 10(-2)M
The compound was stored at -20°C, in the dark because the DMSO is sensitive to the light.

Aug 12

Survivability in molecules which are going to be sensed - Results:
Here are the results of the survivability of Pseudovibrio in media with molecules which are going to be sensed:

Pseudovibrio seems to survive in presence of all concentrations of nitrite, it's affect cells' growth but don't kill Pseudovibrio.
In Lead, big concentrations lead to cells' death, but Pseudovibrio survive very well until a concentration of 5µL.
Cadmium does'nt seem to block cells' growth in concentrations which we want to use.

Survivability in molecules which are going to be sensed:
Ranges of concentrations of molecules that we want to sense were made as described in following tables:

1. Serial dilutions of compound were made



2. 300µL of E.coli BL21 was added in each eppendorf tube.
We had 2 control tubes:
*A negative control: 2,7mL of MB medium + 300µL of compound
*A positive control: 2,7mL of MB medium + 300µL of E.coli BL21
3. Incubation of tubes at 30°C overnight, 200rpm Jul 29

Sensor construction bphR2/PbphR1:
- Plate 20µL from the culture of 27th july on LB-agar-Cam (25 mL LB-Agar + 25µL Chloramphenicol)
- Incubation of plate at 37°C overnight
- A glycerol stock was made with 750ml of culture and 750ml of glycerol 50% and stored at -80°C

Sensor construction bphR2/PbphR1:
- Culturing of one colony bphR1 promoter (BBa_K1155001), received from Paris-Saclay's team, in 5ml LB + 5µL Chloramphenicol (Cam)
- Incubation at 37°C overnight, 200rpm
Interlab study
Week 7
Interlab Study
08.11.2014
08.12.2014
The 4 needed parts are: BBa_J23101, BBa_J23115, BBa_E0240 and BBa_I20260. Corresponding wells were located on 2014 Distribution kit plates and resuspended with 10 µL steril water. That permits to obtain a DNA concentration around 0.2 ng/µl (according to the registry. Solutions were transferred into 1 ml eppendorf tubes and stored at -20°C.
To amplify fragments, a PCR was performed on the 4 constructions, with the mix described on table 1.
Distribution of 49 µl of mix per PCR tube. Application of program IGEM Q5 PCR.
Preparation of a 1% agarose gel: 0.56 g of Top Vision agarose (Thermo Scientific) + 50 ml of TAE 1X.Microwave 30s by 30s until agarose total dissolution. Gel was cooling down until to be lukewarm, one BET drop was added. Gel was loaded with 10µl per sample previously added with 2 µl of loading dye 6X, and 5 µl for ladders. Gel running 45 minutes at 100 mV in TAE 1X buffer.
We expected to obtain one band per PCR sample corresponding to the interesting amplified fragment. For Lane 2, 3 and 6 it was ok. We had the expected profile. By contrast 2 bands were visible, one at the expected size (around 1200 bp) and another around 600 bp. We decided to perform a purification on gel of each bands.
08.13.2014
PCR products were cleaned with the GeneJET purification kit (Thermo Scientific)followed by DNA quantification with the NanoDrop 2000 (Thermo Scientific).
Preparation of a 1% agarose gel: 0.56 g of Top Vision agarose (Thermo Scientific) + 50 ml of TAE 1X. Microwave 30s by 30s until agarose total dissolution Gel was cooling down until to be lukewarm, one BET drop was added. Gel was loaded with 10µl per sample previously added with 2 µl of loading dye 6X, and 5 µl for ladders. Gel running 45 minutes at 100 mV in TAE 1X buffer.
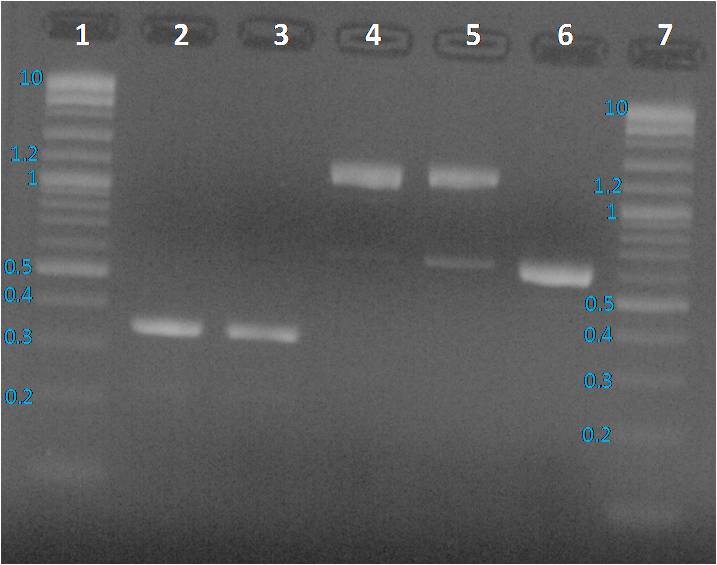
Profiles were same as before PCR clean up. To sequence the amplified parts, we decided to a purification of fragments from the gel as presented below.
Preparation of a 1% agarose gel: 0.56 g of Top Vision agarose (Thermo Scientific) + 50 ml of TAE 1X. Microwave 30s by 30s until agarose total dissolution. Gel was cooling down until to be lukewarm, one BET drop was added. Gel was loaded with 30 µl of BBa_E0240 purified PCR product and BBa_I20260 purified PCR product added with 6 µl of loading dye, at 10µl of the mix per well. Gel running 45 minutes at 100 mV in TAE 1X buffer.
Four pieces of gel were sampled in four tubes to perform a DNA purification from gel.
08.14.2014
To amplify BBa_I20260 and BBa_0240, a PCR was perform with the mix described Table 1. The program IGEM Q5 PCR was applied, see Table 2. PCR products were cleaned with the GeneJET purification kit (Thermo Scientific)followed by DNA quantification with the NanoDrop 2000 (Thermo Scientific).
Preparation of a 1% agarose gel: 0.52 g of Top Vision agarose (Thermo Scientific) + 50 ml of TAE 1X. Microwave 30s by 30s until agarose total dissolution Gel was cooling down until to be lukewarm, one BET drop was added. Gel was loaded with 20 µl of BBa_E0240 PCR product and BBa_I20260 purified PCR product added with 4 µl of loading dye, at 10µl of the mix per well.
Gel running 45 minutes at 100 mV in TAE 1X buffer.
3 pieces of gel were sampled in four tubes to perform a DNA purification from gel. The DNA purification was made with the GeneJET purification kit (Thermo Scientific.
A verification electrophoresis was performed on a 1% agarose gel.
The major part of the DNA was lost during the purification because the amount of was to weak to be purified from gel. So we decide do transform ''E. coli'' to isolate a colony containing the desired plasmid for BBa_E0240 and BBa_I20260.
08.15.2014
BBa_E0240 and BBa_I20260 are respectively into a PSB1C3 and a PSB1K3 vector. In order to select colonies, LB complemented with kanamycin or chloramphenicol are necessary. 200 ml of LB agar medium was prepared with 7 g of LB Agar powder(Sigma) into 200 ml of miliQ water. After complete dissolution, the solution was sterilized in the Tuttmauer 2540ML apparatus, STE 20 minutes at 121°C and EXT+DRY 15 minutes. 50 µl of kanamycin stock solution (25 mg/L) was added to 50 ml of LB agar to pour 2 plates. 150 µl of chloramphenicol stock solution was added in the 150 LB agar remaining ml to pour 6 plates.
The transformation was performed on DH5 alpha ''E. coli'', as followed:
- Remove E. coli competent tubes from -80°C and keep it on ice
- Add 1 µl of template (here solubilized plasmids from the registry distribution kit) and mix gently
- Incubate 10 minutes on ice
- Perform an heat shock 30 seconds at 42°C
- Incubate 2 minutes on ice
- Add 3 ml of LB medium and incubate 60 minutes at 37°C with an agitation at 200 rpm
- Plate 200 µl of BBa_E0240 on a chloramphenicol LB agar plate and BBa_I20260 on a Knamycin LB agar plate
- Incubate plate overnight at 37°C
08.16.2014
Nothing had grown during the night on both plates. Taking into consideration different hypothesis, the transformation was repeated with a change on step 6 and 7. At step 6, only 1 ml of LB medium was added. To plate, 200 µl of BBa_E0240 and BBa_I20260 were plated on 3 plates: LB agar, LB agar kanamycin and LB agar chloramphenicol.
08.17.2014
Colonies had grown on plates as followed:- LB agar: more than 1000 colonies for the two biobricks
- LB agar kanamycin: 10 colonies for BBa_E0240 and >40 colonies for BBa_I20260
- LB agar chloramphenicol: >50 colonies for BBa_E0240 and 3 colonies for BBa_I20260
Plate storage at 4°C.
 "
"









