Team:LMU-Munich/Team/Collaborations
From 2014.igem.org
(→Results:) |
(→Results:) |
||
| Line 96: | Line 96: | ||
tooltip: { | tooltip: { | ||
shared: true, | shared: true, | ||
| - | headerFormat: '<span style="font-size: | + | headerFormat: '<span style="font-size: 13px;font-weight:bold;"> {point.key}</span><br/>' |
}, | }, | ||
Revision as of 16:18, 17 October 2014
Collaborations
Collaborations and the exchange of knowledge were important aspects during our iGEM participation. Therefore we had a intensive collaboration with the iGEM team Groningen (Netherlands) and laid a high priority on the exchange with the Rathenau Institute also located in the Netherlands. In addition we also took part in the Interlab Study of this year´s iGEM competition.
Interlab Study
Background:
The aim of the Interlab study was to evaluate three devices consisting of different Anderson promoters fused to GFP in two different backbones: pSB1C3 and pSB3K3. The devices were distributed by iGEM and the flourescence evaluation method was free of choice. For more details check: Interlab Study@iGEM
The devices:
- Device one: BBa_I20260 consisting of J23101-B0032-E0040-B0015 in the backbone pSB3K3
- Status: ready for evaluation
- Device two: BBa_J23101 + BBa_E0240, each in the backbone pSB1C3
- Status: cloning before evaluation
- Device three: BBa_J23115 + BBa_E0240, each in the backbone pSB1C3
- Status: cloning before evaluation
The cloning:
Devices two and three were built from their individual parts. The plasmids containing the promotors (BBa_J23101 and BBa_J23115) were cut open with SpeI-HF and PstI-HF restriction enzymes. The XbaI and PstI-HF digested GFP (BBa_E0240) was then ligated downstream of the promotors. Both devices were transformed into DH5α, plated out on chloramphenicol [35mg/µl] LB-agar plates and tested via colony-PCR to identify positive clones. For clarification PCR-positive clones were sequenced: device two and device three
Flow cytometry analysis:
For each measurement 50 µl of an overnight culture were first incubated with 1 µl of the membrane stain FM4-64 (final concentration: 4ng/µl) at 37°C for 60 minutes. This is necessary to differentiate between dust or cell particles and intact cells during the measurement. After the incubation, 1 µl of each sample was diluted in 200 µl phosphate buffered saline solution and then evaluated by flow cytometry. In order to generate meaningful results the described measurement were perfomed three times with technical replicates.
For the analysis, all events were gated for a signal in the red channel (which corresponds to intact cells). For those events, the gfp-fluorescence was measured in fluorescence units. The graph below shows the avarage of the geometric mean of the gfp signals from 3 experiments. The empty DH5α strain was used as control for auto fluorescence of the cells.
For details please check our Interlab study submission: LMU-Munich Interlab form
Results:
The results indicate that the promoter BBa_K823005 shows the highest expression of GFP, followed by the device BBa_I20260 (BBa_K823005 + B0032). The lowest GFP expression was measured with the device carrying BBa_K823012.
Discussion
Regarding the measurements from the LMU-iGEM Team of 2012 ([http://parts.igem.org/wiki/index.php?title=Part:BBa_K823012 here]) our generated results go hand in hand with those from 2012 where the activity of several Anderson promoters were measured in B. subtilis. Back then the Anderson promoter BBa_K823005 showed a 5-fold higher (at its maximum) LUMI-read out compared to the BBa_K823012.
The results generated for the interlab study in E. coli show a 10-fold higher expression read out with GFP for BBa_K823005 compared to BBa_K823012. Having a closer look at the [http://parts.igem.org/Part:BBa_I20260 BBa_I20260] device, the insertion of a weaker RBS binding site (as described [http://parts.igem.org/Part:BBa_B0032 here]) might explain the lower expression of GFP compared to BBa_K823005, despite containing the same promotor. In addition, the different backbones and therefore the diverse genetic environments and copy numbers might also have an influence on the expression level and should be closer investigated for further conclusions.
Outlook
The LMU-iGEM team is looking forward to the presentation of iGEM HQ results, were we hope to see a mixture of different methods utilized - hopefully with similar outcomes. We are curious how the absolute and relative results might differ; this might be a milestone towards ensuring reproducibility of scientific data produced by a huge number of different labs and methods.
Collaboration with Team Groningen
Work done in our Lab
On Monday 13th of October we received parts to be evaluated in Bacillus subtilis from the iGEM Team Groningen. The goal was to evaluate Promoters fused to a super-folded Green Fluorescent Protein (sfGFP) towards their activity in B. subtilis. In order to do so we had to bring the BioBricks into our integrative BioBrick compatible backbone pBS1C (LINK). The final analysis was done using fluorescent-activated cell sorting (FACS).
Results:
A proposal of the German iGEM Teams concerning Intellectual Property
During the meetup of the German iGEM teams from 23rd to 25th May a workshop also took place in which amongst others we discussed the topic of bioethics. Moral questions were addressed, regarding the value of life and human influence on it, as well as questions dealing with the possible socioeconomic effects of synthetic biology.
Especially the topic of an open source vs. patent controlled field accounted for a large part of the discussion. During the discussion one student brought up the point that the legal status of parts in the parts registry remains unclear and that there are parts (e.g. [http://parts.igem.org/Part:BBa_K180009 BBa_K180009]) where it only becomes clear upon a closer that the rights are company–owned. This issue (that the legal status of parts in the registry remains uncertain) is also mentioned in a recent article published by Nature [1]:
"[N]o one can say with any certainty how many of these parts are themselves entirely free of patent claims."
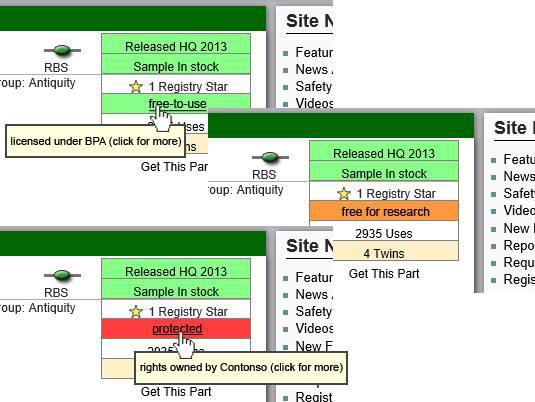
We, the German iGEM teams, therefore like to suggest the addition of a new feature to the parts registry:
A dedicated data field of license information for each BioBrick part.
For the implementation, we propose to introduce two new fields to the BioBrick part entries in the registry:
- A string property "LicenseInfo"
- A traffic light property (grey, green, yellow, red) to indicate the level of legal protection (unknown, BPA-like, free for research purposes, heavily protected)
Implementing this feature would in our opinion further clarify and extend the parts info, provide a machine-readable format and thus improve future entries. With the emerging Entrepreneurship track and applications getting closer to industrial realization, the legal status becomes more and more relevant. It would also raise awareness to the topic of the legal status of parts. This could lead to a debate which could further promote the idea of open source, ideally. At the same time we hope that the examination of most parts will show that they are indeed free of restrictive legal protections.
- The German iGEM Teams,
Aachen Berlin Bielefeld-CeBiTec Braunschweig Freiburg Goettingen Hannover Heidelberg LMU-Munich Marburg Saarland Tuebingen TU Darmstadt
References:
- [http://www.nature.com/news/synthetic-biology-cultural-divide-1.15149| Bryn Nelson ‘Synthetic Biology: Cultural Divide ’, Nature 509, 152–154, 08 May 2014]
Collaboration with Team Virginia
The global synthetic biology awareness and acceptance survey designed by the Virginia iGEM-team was a great opportunity to generate a lot of data,
since more than 40 teams participated! This also perfectly encapsulates the spirit of iGEM, since this is probably the biggest
collaboration in the history of the competition.

Hi there!
Welcome to our Wiki! I'm BaKillus, the pathogen-hunting microbe, and I'll guide you on this tour through our project. If you want to learn more about a specific step, you can simply close the tour and come back to it anytime you like. So let's start!

What's the problem?
First of all, what am I doing here? The problem is, pathogenic bacteria all around the world are becoming more and more resistant against antimicrobial drugs. One major reason for the trend is the inappropriate use of drugs. With my BaKillus super powers, I want to reduce this misuse and thus do my part to save global health.

Sensing of pathogens
To combat the pathogenic bacteria, I simply eavesdrop on their communication. Bacteria talk with each other via quorum sensing systems, which I use to detect them and trigger my responses.

Adhesion
The more specific and effective I can use my powers, the lower the danger is of provoking new resistance development. So I catch pathogens whenever I get hold of them and stick to them until my work is done.

Killing
Talking about my work - killing pathogens is finally what I am made for. In response to quorum sensing molecules of the pathogens, I export a range of antimicrobial substances leading to dissipation of biofilms and the killing of the targeted bacteria.

Suicide switch
When the job is done and all the bad guys are finished, you don't need a super hero anymore. So after fulfilling my work I say goodbye to the world by activating my suicide switch.

Application
Of course I'm not only a fictional hero, but a very real one. In two different prototypes, I could be used for diagnosis or treatment of pathogen-caused diseases. However, there is still a whole lot of regulational and economical questions that have to be answered before.

See you!
So now you know my short story - and it is time for me to return to my fight for a safer world. Feel free to take a closer look on my super powers, the process of my development or the plans for a medical application.
 "
"