Team:Freiburg/Content/Results/Vector
From 2014.igem.org
Mirja Harms (Talk | contribs) |
|||
| (48 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
| + | <!-- hier ändern https://2014.igem.org/Team:Freiburg/Content/Results/Vector | ||
| + | class="img-no-border" class="img-no-pad" class="fig-full-width" | ||
| + | <a href="https://2014.igem.org/Team:Freiburg/Project/Receptor">Read More about the Receptor</a> | ||
| + | <h4 id="MirjaHarms_Red_light"> SEAP assay Red light system/ comparison different light boxes</h4>--> | ||
<html lang="en"> | <html lang="en"> | ||
<head> | <head> | ||
| Line 5: | Line 9: | ||
</head> | </head> | ||
<body> | <body> | ||
| + | <div class="row category-row"> | ||
| + | <div class="col-sm-6"> | ||
| + | <div class="container-fluid" style="float: left"> | ||
| + | <div style="position: relative; float: right; margin-top: 4px;"> | ||
| + | <a href="https://2014.igem.org/Team:Freiburg/Results">Go back to the Summary from our Results</div> | ||
| + | <div style="position: relative; float: left;"> <img class="img-no-border" style="max-width: 50px; margin-top:5px;" src=" https://static.igem.org/mediawiki/2014/4/44/Freiburg2014_Navigation_Arrow_rv.png"> <!-- Pfeil rv--></a></div> | ||
| + | </div> | ||
| + | </div> | ||
| + | <div class="col-sm-6"> | ||
| + | <div class="container-fluid" style="float: right"> | ||
| + | <div style="position: relative; float: left; margin-top: 4px;"> | ||
| + | |||
| + | <a href="https://2014.igem.org/Team:Freiburg/Results/Receptor">Go on and have a look at our Receptor</div> | ||
| + | <div style="position: relative; float: right;"> <img class="img-no-border" style="max-width: 50px; margin-top:5px;" src=" https://static.igem.org/mediawiki/2014/9/95/Freibur2014_pfeilrechts.png"> <!-- Pfeil fw--></a></div> | ||
| + | </div> | ||
| + | </div> | ||
| + | </div> | ||
<section id="Vector"> | <section id="Vector"> | ||
<h1>The Vector</h1> | <h1>The Vector</h1> | ||
| + | </section> | ||
| + | <section> | ||
<h2 id="Vector-Introduction">Introduction</h2> | <h2 id="Vector-Introduction">Introduction</h2> | ||
<div class="row category-row"> | <div class="row category-row"> | ||
<div class="col-sm-6"> | <div class="col-sm-6"> | ||
| - | <p>We | + | <p>One important part in our system is the viral vector we use for transferring genes into target cells. We succeeded to stably integrate our gene of interest into the genome of mammalian target cells by using a vector derived from the murine leukemia virus. The advantages of this vector are: its specificity for murine cells, making the viral work safe and easy; a very high efficiency for infection; and the ability of stable gene transfer into target genomes. Here we present results on these three qualities of our viral vector and how we optimized virus production and cell infection.</p> |
</div> | </div> | ||
<div class="col-sm-6"> | <div class="col-sm-6"> | ||
<figure> | <figure> | ||
| - | + | <style scoped> #svg-results-vector {padding-top: 50%; margin-left: auto; margin-right: auto; width: 80%;</style> | |
| + | </html>{{:Team:Freiburg/svg/results_vector.svg}}<html> | ||
<figcaption> | <figcaption> | ||
| - | <p class="header"> | + | <p class="header"></p> |
</figcaption> | </figcaption> | ||
</figure> | </figure> | ||
</div> | </div> | ||
</div> | </div> | ||
| - | + | </section> | |
| - | <h2 id="Vector-Stable-Integration">Stable | + | |
| + | <section> | ||
| + | <h2 id="Vector-Stable-Integration">Stable Integration of Target Genes into the Genome</h2> | ||
<p> | <p> | ||
| - | Our viral vector derives from the murine leukemia virus and since this virus is a retrovirus, it stably integrates our gene of interest into the target genomes. | + | Our viral vector derives from the murine leukemia virus and since this virus is a retrovirus, it stably integrates our gene of interest into the target genomes. Any gene of interest can be delivered. We transferred genes for different fluorescent proteins into murine cell lines (Fig. 1). |
</p> | </p> | ||
| - | <figure> | + | <figure class="fig-full-width"> |
| - | + | <a href="https://static.igem.org/mediawiki/2014/1/10/Freiburg2014-10-06_stable_integration_different_colours.png"> <!-- ORGINAL --> | |
| + | <img class="img-no-pad" src="https://static.igem.org/mediawiki/2014/c/c0/Freiburg2014-10-06_stable_integration_different_colours_Thumbnail.jpg"> <!-- Thumbnail --> | ||
| + | </a> | ||
<figcaption> | <figcaption> | ||
| - | <p class="header"> | + | <p class="header">Figure 1: Murine NIH3T3 cells infected with our viral vector</p> |
| - | + | <p class="desc">The vector contained different fluorescent markers: (A) EGFP, (B) mKO, (C) mKate that were stably integrated into the genome. <a href="https://2014.igem.org/Team:Freiburg/Notebook/Labjournal#Mouse_colours">Labjournal</a></p> | |
</figcaption> | </figcaption> | ||
</figure> | </figure> | ||
<p> | <p> | ||
| - | In addition, we demonstrated the capability of stable gene integration by creating an EGFP cell line | + | In addition, we demonstrated the capability of stable gene integration by creating an EGFP cell line and assessing the fraction of fluorescent cells over several passages. Two days after virus infection, we selected for cells displaying green fluorescence by a cell sorter. Afterwards, we analyzed the EGFP cell line for several weeks by flow cytometry. Sustained expression of EGFP indicates stable integration into the genome of the original cell line (Fig. 2). |
</p> | </p> | ||
| - | <figure> | + | <figure class="fig-full-width"> |
| - | + | <a href="https://static.igem.org/mediawiki/2014/c/c9/Freiburg2014-09-06_stable_EGPF_integration_diagram_and_facs.png"> <!-- ORGINAL --> | |
| + | <img src="https://static.igem.org/mediawiki/2014/c/c9/Freiburg2014-09-06_stable_EGPF_integration_diagram_and_facs.png"> <!-- Thumbnail --> | ||
| + | </a> | ||
<figcaption> | <figcaption> | ||
| - | <p class="header"></p> | + | <p class="header">Figure 2: Stable integration of EGFP into the genome of the target cells.</p> |
| - | <p class="desc"></p> | + | <p class="desc">(A) Histogram of fluorescence intensity at the first sorting step. (B) Histogram of fluorescence intensity at passage xx after sorting. (C) The fraction of fluorescent cells stays constant for many passages. <a href="https://2014.igem.org/Team:Freiburg/Notebook/Labjournal#EGFP_stable_integration">Labjournal</a></p> |
</figcaption> | </figcaption> | ||
</figure> | </figure> | ||
| Line 50: | Line 80: | ||
<div class="col-sm-6"> | <div class="col-sm-6"> | ||
<p> | <p> | ||
| - | For optimal integration of the target gene, | + | For optimal integration of the target gene, we tested how long the viral vector need for transducing murine cells. We incubated murine cells (NIH3T3) with the virus for different time intervals, washed the virus away, and counted fluorescent cells after 48 h. A high transduction rate was already reached after incubating the cells for five hours with the viral particles (Fig. 3). |
</p> | </p> | ||
</div> | </div> | ||
| Line 56: | Line 86: | ||
<div class="col-sm-6"> | <div class="col-sm-6"> | ||
<figure> | <figure> | ||
| - | + | <a href="https://static.igem.org/mediawiki/2014/9/96/Integration_time.png"> <!-- ORGINAL --> | |
| + | <img src="https://static.igem.org/mediawiki/2014/9/96/Integration_time.png"> <!-- Thumbnail --> | ||
| + | </a> | ||
<figcaption> | <figcaption> | ||
| - | <p class="header"> | + | <p class="header">Figure 3: Integration time of our viral vector into murine cells.</p> |
| - | <p class="desc"></p> | + | <p class="desc">Murine NIH3T3 cells were infected with MuLV EGFP and washed at distinct time points. <a href="https://2014.igem.org/Team:Freiburg/Notebook/Labjournal#integration_time">Labjournal</a></p> |
</figcaption> | </figcaption> | ||
</figure> | </figure> | ||
| Line 66: | Line 98: | ||
<p> | <p> | ||
| - | + | To sort the cells that stably integrated the gene of interest into the genome, the viral vector contained a marker, e.g. the gene for a fluorescent protein. But for future experiments, an additional target gene would be placed into the virus cargo, separated from the marker by an internal ribosome entry site (IRES) for selection or sorting. We tested the expression strength of EGFP in pMIG constructs containing only the marker in comparison to constructs containing the target gene upstream of an IRES and EGFP as a marker (Fig. 4). In the construct combining the target gene with the marker, EGFP expression is lower; a possible explanation is the lower rate of translation initiation by the IRES compared to the cap at the 5' end of the mRNA. If only the IRES but no additional target gene is present upstream of EGFP, expression of EGFP is high because the first ATG after the 5' end of the mRNA is the start codon of EGFP. | |
</p> | </p> | ||
| - | <figure> | + | <figure class="fig-full-width"> |
| - | + | <a href="https://static.igem.org/mediawiki/2014/2/20/Freiburg2014-10-02_pMIG_different_consctructs.JPG"> <!-- ORGINAL --> | |
| - | + | <img class="img-no-pad" src="https://static.igem.org/mediawiki/2014/2/20/Freiburg2014-10-02_pMIG_different_consctructs.JPG"> <!-- Thumbnail --> | |
| - | < | + | </a> |
| - | + | ||
| - | </ | + | |
</figure> | </figure> | ||
| - | <figure> | + | <figure class="fig-full-width"> |
| - | <img src="https://static.igem.org/mediawiki/2014/ | + | <a href="https://static.igem.org/mediawiki/2014/b/b2/Freiburg2014-10-02_plasmid_map_pMIG_constructs_skizze.JPG"> <!-- ORGINAL --> |
| + | <img class="img-no-pad" src="https://static.igem.org/mediawiki/2014/b/b2/Freiburg2014-10-02_plasmid_map_pMIG_constructs_skizze.JPG"> <!-- Thumbnail --> | ||
| + | </a> | ||
<figcaption> | <figcaption> | ||
| - | <p class="header"> | + | <p class="header">Figure 4: Expression strength of EGFP sorting marker with and without an IRES.</p> |
| - | <p class="desc"></p> | + | <p class="desc">(A) Cargo contains only EGFP, (B) cargo contains EGFP downstream of an IRES, (C) cargo contains a gane of interest, an IRES, and the EGFP marker. <a href="https://2014.igem.org/Team:Freiburg/Notebook/Labjournal#pMIG_constructs">Labjournal</a></p> |
</figcaption> | </figcaption> | ||
</figure> | </figure> | ||
| - | <blockquote><strong> | + | <blockquote><strong>With our viral vector any gene of interest can be delivered to generate new stable cell lines. The advantages of generating cell lines with our vector are its efficiency and its specificity making handling easy and safe! It also takes only one week!</strong></blockquote> |
| - | + | ||
| - | <h2 id="Vector-High-Efficiency">High | + | </section> |
| + | |||
| + | <section> | ||
| + | <h2 id="Vector-High-Efficiency">High Efficiency</h2> | ||
<div class="row category-row"> | <div class="row category-row"> | ||
<div class="col-sm-6"> | <div class="col-sm-6"> | ||
| - | <p> | + | <p>We increased the transduction efficiency of our virus to over 80% in murine cell lines and achieved high infection rates in other cell lines expressing the murine CAT-1 receptor. |
| + | </p> | ||
| + | <p> | ||
| + | Protocols for virus production and target cell transduction were optimized. As producer cell line for viral particles we used Phoenix cells which stably express most of the viral genes. Before transfection with pMIG, the final plasmid to initiate virus production, we split the Phoenix cells several times to increase viability and push them into the exponential growth phase during production of the viral vector. In addition, supernatant containing the vector was harvested at distinct time points optimized for high viral titer. Target cells were infected by viral supernatant with polybrene which increases the probability for particles to reach their target cells. Since murine leukemia viruses only infect dividing cells, the phase of maximum division rate of the target cells was matched to the time point of infection. | ||
| + | </p> | ||
| + | <p> | ||
| + | Under these optimized conditions, the efficiency for transducing murine cell lines with our viral vector was over 80% (Fig. 5, Fig. 6). Since we had only 16% transduction efficiency at the beginning of the project, we would consider this a significant advance. To our knowledge, an optimized infection protocol with murine leukemia virus has not been published to date. The exact procedure can be found here (<a href="https://2014.igem.org/Team:Freiburg/Notebook/Methods#Notebook-Methods-Cell-Culture-Virus-Production">Methods</a>). | ||
| + | </p> | ||
| + | |||
| + | <figure> | ||
| + | <a href="https://static.igem.org/mediawiki/2014/0/0e/Freiburg2014-10-06_dilution_concentration_virus.png"> <!-- ORGINAL --> | ||
| + | <img src="https://static.igem.org/mediawiki/2014/0/0e/Freiburg2014-10-06_dilution_concentration_virus.png"> <!-- Thumbnail --> | ||
| + | </a> | ||
| + | <figcaption> | ||
| + | <p class="header">Figure 7: Impact of dilution and concentration of the virus on transduction efficiency.</p> | ||
| + | <p class="desc">Concentration of the virus was obtained by incubation with chondroitin sulfate and polybrene, centrifugation and resuspension in reduced volume (see protocols (<a href="https://2014.igem.org/Team:Freiburg/Notebook/Methods#Notebook-Methods-Cell-Culture-Virus-Concentration">Methods</a>)).</p> | ||
| + | </figcaption> | ||
| + | </figure> | ||
| + | <figure> | ||
| + | <a href="https://static.igem.org/mediawiki/2014/6/67/HWZ.png"> <!-- ORGINAL --> | ||
| + | <img src="https://static.igem.org/mediawiki/2014/6/67/HWZ.png"> <!-- Thumbnail --> | ||
| + | </a> | ||
| + | <figcaption> | ||
| + | <p class="header">Figure 8: Decay of viral particles.</p> | ||
| + | <p class="desc">The virus was first incubated at 37°C and then added to the NIH3T3 cell culture. Transduction efficiency was measured after 48 hours. <a href="https://2014.igem.org/Team:Freiburg/Notebook/Labjournal#Virus_Decay">Labjournal</a></p> | ||
| + | </figcaption> | ||
| + | </figure> | ||
</div> | </div> | ||
| + | |||
<div class="col-sm-6"> | <div class="col-sm-6"> | ||
<figure> | <figure> | ||
| - | <img src="https://static.igem.org/mediawiki/2014/0/0d/Freiburg2014-10-02_transduction_efficiency_in_pictures.JPG"> | + | <a href="https://static.igem.org/mediawiki/2014/0/0d/Freiburg2014-10-02_transduction_efficiency_in_pictures.JPG"> <!-- ORGINAL --> |
| + | <img class="img-no-pad" src="https://static.igem.org/mediawiki/2014/0/0d/Freiburg2014-10-02_transduction_efficiency_in_pictures.JPG"> <!-- Thumbnail --> | ||
| + | </a> | ||
<figcaption> | <figcaption> | ||
| - | <p class="header"> | + | <p class="header">Figure 5: Transduction efficiency increased after optimation of infection protocol.</p> |
| - | <p class="desc"></p> | + | <p class="desc">Murine cells were infected with MuLV transferring the gene for EGFP, (A) transduction efficiency in July was 16%, (B) transduction efficiency in August was 30%, (C) transduction efficiency in September was 45%, (D) transduction efficiency in October was 85%.</p> |
</figcaption> | </figcaption> | ||
</figure> | </figure> | ||
| + | |||
<figure> | <figure> | ||
| - | <img src="https://static.igem.org/mediawiki/2014/4/44/Freiburg2014-10-02_transduction_efficiency_before_and_after.JPG"> | + | <a href="https://static.igem.org/mediawiki/2014/4/44/Freiburg2014-10-02_transduction_efficiency_before_and_after.JPG"> <!-- ORGINAL --> |
| + | <img src="https://static.igem.org/mediawiki/2014/4/44/Freiburg2014-10-02_transduction_efficiency_before_and_after.JPG"> <!-- Thumbnail --> | ||
| + | </a> | ||
<figcaption> | <figcaption> | ||
| - | <p class="header"> | + | <p class="header">Figure 6: Optimization of the virus harvesting and infection prococol throughout the course of our project.</p> |
| - | <p class="desc"></p> | + | <p class="desc">Murine cells were infected with MuLV transferring EGFP. Transduction efficiency increased from 16% of over 85%.</p> |
</figcaption> | </figcaption> | ||
</figure> | </figure> | ||
| - | + | ||
| - | + | <p>Since the viral titer has direct impact on the infection rate of target cells, we assessed the transduction efficiency of the viral particles after dilution and concentration. Concentration was acheived by incubating the viral supernatant with hexadimethrine bromide (polybrene) as well as with chondroitin sulfate, leading to formation of complexes that were big enough for pelleting by centrifugation. Pellets contained the viral particles and were resuspended in a small volume of growth medium. Infecting murine cells with the concentrated vector lead to a higher transduction rate compared to the unconcentrated virus or the diluted virus (Fig. 7). | |
| - | + | </p> | |
| - | + | <p>In addition, we determined the decay curve of the viral particles. Incubation at 37°C leads to a strong decrease of the infection rate with a half time of approximately 6.5 hours (Fig. 8). This short half life provides an enhanced safety regarding work and handling of our viral vector. | |
| - | + | </p> | |
| - | + | ||
| - | </ | + | |
| - | + | ||
</div> | </div> | ||
| - | + | </div> | |
| + | |||
| - | <blockquote><strong> | + | <blockquote><strong>Transduction efficiency of our viral vector in murine cells was optimized to over 85%!</strong></blockquote> |
| + | </section> | ||
| + | <section> | ||
| + | <h2 id="Vector-Specificity-and-Safety">Specificity and Safety of MuLV</h2> | ||
| + | <!-- Half life time of MuLV --> | ||
<div class="row category-row"> | <div class="row category-row"> | ||
<div class="col-sm-6"> | <div class="col-sm-6"> | ||
| - | <p> | + | <p>An important aspect for the function of our system as well as for its safety is the specificity of the vector regarding infection of different kind of cells. Cells that do not have this specific receptor are not targeted by the vector (Fig. 9; Fig. 10). To test the specificity of the system, different kinds of cells were incubated with the vector containing EGFP. EGFP is stably integrated by the system. We tested human cell lines, human embryonic kidney cells as well as human lung epithel carcinoma cells and breast cancer cells, for their susceptibility of being infected by the vector. In none of them, green fluorescent cells were observed (Fig. 10). Murine cells (NIH3T3) that express the mouse CAT-1 receptor were tested as a positive control (Fig. 10). Quantification by flow cytometry indeed showed that human cells were not transduced, whereas a large fraction of the mouse control cells were transduced with the same virus batch (Fig. 11, Fig. 12). 9E-G).</p> |
| - | + | ||
</div> | </div> | ||
| - | + | ||
<div class="col-sm-6"> | <div class="col-sm-6"> | ||
| - | |||
| - | |||
<figure> | <figure> | ||
| - | <img src="https://static.igem.org/mediawiki/2014/ | + | <a href="https://static.igem.org/mediawiki/2014/1/1e/Freiburg2014-10-02_results_skizze_specificity.png"> <!-- ORGINAL --> |
| + | <img src="https://static.igem.org/mediawiki/2014/1/1e/Freiburg2014-10-02_results_skizze_specificity.png"> <!-- Thumbnail --> | ||
| + | </a> | ||
<figcaption> | <figcaption> | ||
| - | + | <p class="header">Figure 9: Specificity of the virus.</p> | |
| - | + | <p class="desc">The virus uses the murine mCAT-1 receptor as an entry site into the cell. Therefore, it can specifically transduce mouse cells (e.g. NIH3T3), but not cells lines from other species (e.g. the human embryonic kidney (HEK-293T) cells).</p> | |
</figcaption> | </figcaption> | ||
</figure> | </figure> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
</div> | </div> | ||
</div> | </div> | ||
| - | <figure> | + | |
| - | + | <figure class="fig-full-width"> | |
| - | + | <a href="https://static.igem.org/mediawiki/2014/7/79/Freiburg2014_Results_Specificity_MuLV_microscopy.jpg"> <!-- ORGINAL --> | |
| - | + | <img class="img-no-pad" src="https://static.igem.org/mediawiki/2014/7/79/Freiburg2014_Results_Specificity_MuLV_microscopy.jpg"> <!-- Thumbnail --> | |
| - | + | </a> | |
| - | + | <figcaption> | |
| + | <p class="header">Figure 10: Infection of different cell lines with the viral vector derived from the murine leukemia virus. </p> | ||
| + | <p class="desc">Cells that were infected by viral particles express EGFP. <a href="<a href=https://2014.igem.org/Team:Freiburg/Notebook/Labjournal#Specificity_pictures">Labjournal</a></p> | ||
| + | </figcaption> | ||
| + | </figure> | ||
| + | |||
<div class="row category-row"> | <div class="row category-row"> | ||
<div class="col-sm-6"> | <div class="col-sm-6"> | ||
<figure> | <figure> | ||
| - | <img src="https://static.igem.org/mediawiki/2014/4/45/Freiburg2014-09-10_FACS-specificity-HEK-MOUSE.png"> | + | <a href="https://static.igem.org/mediawiki/2014/4/45/Freiburg2014-09-10_FACS-specificity-HEK-MOUSE.png"> <!-- ORGINAL --> |
| + | <img src="https://static.igem.org/mediawiki/2014/4/45/Freiburg2014-09-10_FACS-specificity-HEK-MOUSE.png"> <!-- Thumbnail --> | ||
| + | </a> | ||
<figcaption> | <figcaption> | ||
| - | <p class="header">Fig. | + | <p class="header">Fig. 11: FACS analysis of murine cells (NIH3T3) incubated with the mCAT-1 specific viral vector</p> |
| - | <p class="desc"> | + | <p class="desc">Murine cells were transduced with MuLV EGFP and analysed with flow cytometry after 48 hours of incubation.</p> |
</figcaption> | </figcaption> | ||
</figure> | </figure> | ||
| Line 175: | Line 232: | ||
<div class="col-sm-6"> | <div class="col-sm-6"> | ||
<figure> | <figure> | ||
| - | <img src="https://static.igem.org/mediawiki/2014/5/5a/Freiburg2014-09-10_cell-line-specificity.png"> | + | <a href="https://static.igem.org/mediawiki/2014/5/5a/Freiburg2014-09-10_cell-line-specificity.png"> <!-- ORGINAL --> |
| + | <img src="https://static.igem.org/mediawiki/2014/5/5a/Freiburg2014-09-10_cell-line-specificity.png"> <!-- Thumbnail --> | ||
| + | </a> | ||
<figcaption> | <figcaption> | ||
| - | <p class="header">Fig. | + | <p class="header">Fig. 12: Infection of different cell lines with the viral vector derived from the murine leukemia virus.</p> |
| - | <p class="desc">In order to investigate the specificity of the viral vector, two human cell lines, human embryonic kidney cells as well as lung epithel carcinoma cells, were infected and the percentage of cells expressing EGFP was analyzed by flow cytometry.</p> | + | <p class="desc">In order to investigate the specificity of the viral vector, two human cell lines, human embryonic kidney cells as well as lung epithel carcinoma cells, were infected and the percentage of cells expressing EGFP was analyzed by flow cytometry. <a href="<a href=https://2014.igem.org/Team:Freiburg/Notebook/Labjournal#Specificity_pictures">Labjournal</a></p> |
</figcaption> | </figcaption> | ||
</figure> | </figure> | ||
| Line 184: | Line 243: | ||
</div> | </div> | ||
| - | <blockquote><strong>The vector derived from the murine leukemia virus is specific for the murine CAT-1 receptor. Therefore it is not able to infect human cell lines!</blockquote> | + | <blockquote><strong>The vector derived from the murine leukemia virus is specific for the murine CAT-1 receptor. Therefore it is not able to infect human cell lines making handling easy and safe!</strong></blockquote> |
</section> | </section> | ||
| - | + | <div class="row category-row"> | |
| - | + | <div class="col-sm-6"> | |
| - | + | <div class="container-fluid" style="float: left"> | |
| - | + | <div style="position: relative; float: right; margin-top: 4px;"> | |
| + | <a href="https://2014.igem.org/Team:Freiburg/Results">Go back to the Summary from our Results</div> | ||
| + | <div style="position: relative; float: left;"> <img class="img-no-border" style="max-width: 50px; margin-top:5px;" src=" https://static.igem.org/mediawiki/2014/4/44/Freiburg2014_Navigation_Arrow_rv.png"> <!-- Pfeil rv--></a></div> | ||
| + | </div> | ||
| + | </div> | ||
| + | <div class="col-sm-6"> | ||
| + | <div class="container-fluid" style="float: right"> | ||
| + | <div style="position: relative; float: left; margin-top: 4px;"> | ||
| + | |||
| + | <a href="https://2014.igem.org/Team:Freiburg/Results/Receptor">Go on and have a look at our Receptor</div> | ||
| + | <div style="position: relative; float: right;"> <img class="img-no-border" style="max-width: 50px; margin-top:5px;" src=" https://static.igem.org/mediawiki/2014/9/95/Freibur2014_pfeilrechts.png"> <!-- Pfeil fw--></a></div> | ||
| + | </div> | ||
| + | </div> | ||
| + | </div> | ||
Latest revision as of 03:06, 18 October 2014
The Vector
Introduction
One important part in our system is the viral vector we use for transferring genes into target cells. We succeeded to stably integrate our gene of interest into the genome of mammalian target cells by using a vector derived from the murine leukemia virus. The advantages of this vector are: its specificity for murine cells, making the viral work safe and easy; a very high efficiency for infection; and the ability of stable gene transfer into target genomes. Here we present results on these three qualities of our viral vector and how we optimized virus production and cell infection.
Stable Integration of Target Genes into the Genome
Our viral vector derives from the murine leukemia virus and since this virus is a retrovirus, it stably integrates our gene of interest into the target genomes. Any gene of interest can be delivered. We transferred genes for different fluorescent proteins into murine cell lines (Fig. 1).

Figure 1: Murine NIH3T3 cells infected with our viral vector
The vector contained different fluorescent markers: (A) EGFP, (B) mKO, (C) mKate that were stably integrated into the genome. Labjournal
In addition, we demonstrated the capability of stable gene integration by creating an EGFP cell line and assessing the fraction of fluorescent cells over several passages. Two days after virus infection, we selected for cells displaying green fluorescence by a cell sorter. Afterwards, we analyzed the EGFP cell line for several weeks by flow cytometry. Sustained expression of EGFP indicates stable integration into the genome of the original cell line (Fig. 2).

Figure 2: Stable integration of EGFP into the genome of the target cells.
(A) Histogram of fluorescence intensity at the first sorting step. (B) Histogram of fluorescence intensity at passage xx after sorting. (C) The fraction of fluorescent cells stays constant for many passages. Labjournal
For optimal integration of the target gene, we tested how long the viral vector need for transducing murine cells. We incubated murine cells (NIH3T3) with the virus for different time intervals, washed the virus away, and counted fluorescent cells after 48 h. A high transduction rate was already reached after incubating the cells for five hours with the viral particles (Fig. 3).
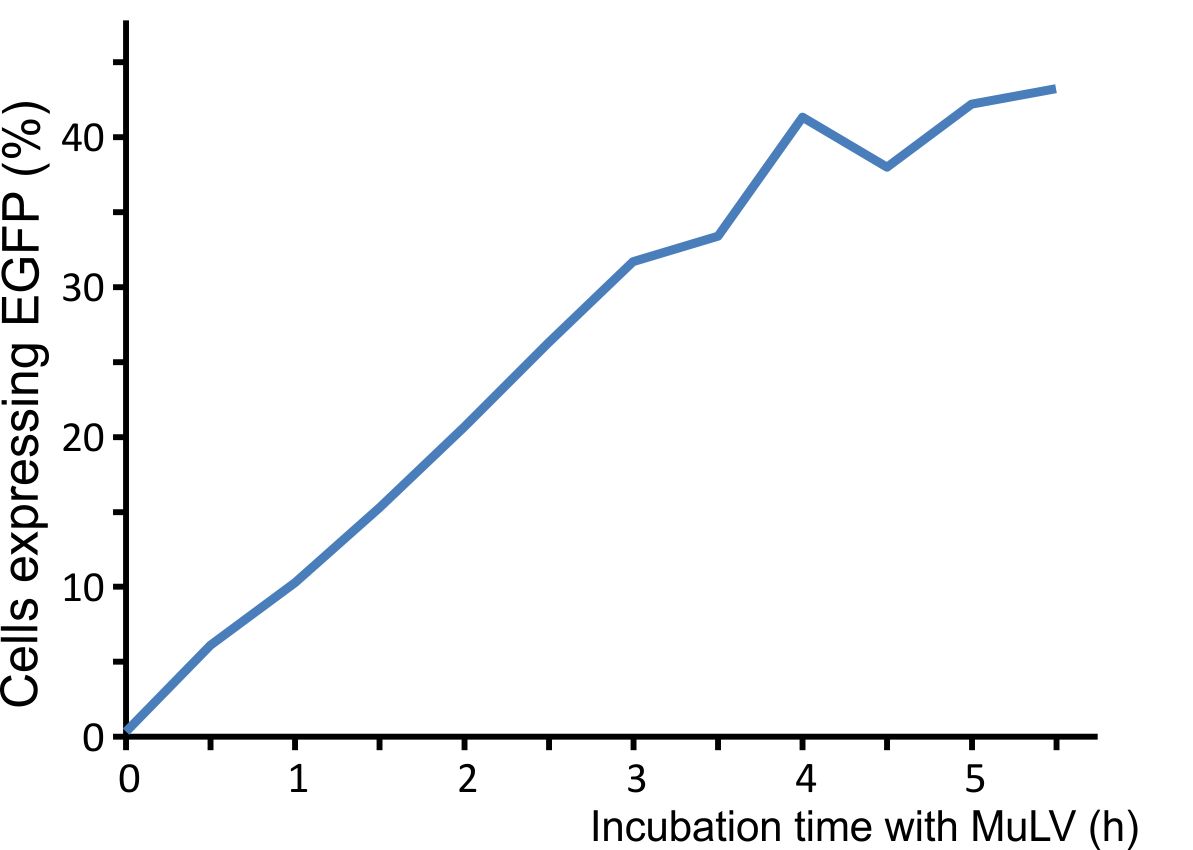
Figure 3: Integration time of our viral vector into murine cells.
Murine NIH3T3 cells were infected with MuLV EGFP and washed at distinct time points. Labjournal
To sort the cells that stably integrated the gene of interest into the genome, the viral vector contained a marker, e.g. the gene for a fluorescent protein. But for future experiments, an additional target gene would be placed into the virus cargo, separated from the marker by an internal ribosome entry site (IRES) for selection or sorting. We tested the expression strength of EGFP in pMIG constructs containing only the marker in comparison to constructs containing the target gene upstream of an IRES and EGFP as a marker (Fig. 4). In the construct combining the target gene with the marker, EGFP expression is lower; a possible explanation is the lower rate of translation initiation by the IRES compared to the cap at the 5' end of the mRNA. If only the IRES but no additional target gene is present upstream of EGFP, expression of EGFP is high because the first ATG after the 5' end of the mRNA is the start codon of EGFP.


Figure 4: Expression strength of EGFP sorting marker with and without an IRES.
(A) Cargo contains only EGFP, (B) cargo contains EGFP downstream of an IRES, (C) cargo contains a gane of interest, an IRES, and the EGFP marker. Labjournal
With our viral vector any gene of interest can be delivered to generate new stable cell lines. The advantages of generating cell lines with our vector are its efficiency and its specificity making handling easy and safe! It also takes only one week!
High Efficiency
We increased the transduction efficiency of our virus to over 80% in murine cell lines and achieved high infection rates in other cell lines expressing the murine CAT-1 receptor.
Protocols for virus production and target cell transduction were optimized. As producer cell line for viral particles we used Phoenix cells which stably express most of the viral genes. Before transfection with pMIG, the final plasmid to initiate virus production, we split the Phoenix cells several times to increase viability and push them into the exponential growth phase during production of the viral vector. In addition, supernatant containing the vector was harvested at distinct time points optimized for high viral titer. Target cells were infected by viral supernatant with polybrene which increases the probability for particles to reach their target cells. Since murine leukemia viruses only infect dividing cells, the phase of maximum division rate of the target cells was matched to the time point of infection.
Under these optimized conditions, the efficiency for transducing murine cell lines with our viral vector was over 80% (Fig. 5, Fig. 6). Since we had only 16% transduction efficiency at the beginning of the project, we would consider this a significant advance. To our knowledge, an optimized infection protocol with murine leukemia virus has not been published to date. The exact procedure can be found here (Methods).

Figure 7: Impact of dilution and concentration of the virus on transduction efficiency.
Concentration of the virus was obtained by incubation with chondroitin sulfate and polybrene, centrifugation and resuspension in reduced volume (see protocols (Methods)).

Figure 8: Decay of viral particles.
The virus was first incubated at 37°C and then added to the NIH3T3 cell culture. Transduction efficiency was measured after 48 hours. Labjournal
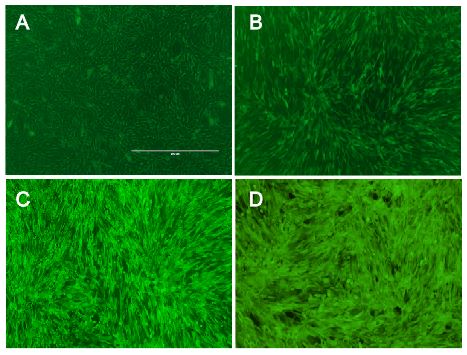
Figure 5: Transduction efficiency increased after optimation of infection protocol.
Murine cells were infected with MuLV transferring the gene for EGFP, (A) transduction efficiency in July was 16%, (B) transduction efficiency in August was 30%, (C) transduction efficiency in September was 45%, (D) transduction efficiency in October was 85%.

Figure 6: Optimization of the virus harvesting and infection prococol throughout the course of our project.
Murine cells were infected with MuLV transferring EGFP. Transduction efficiency increased from 16% of over 85%.
Since the viral titer has direct impact on the infection rate of target cells, we assessed the transduction efficiency of the viral particles after dilution and concentration. Concentration was acheived by incubating the viral supernatant with hexadimethrine bromide (polybrene) as well as with chondroitin sulfate, leading to formation of complexes that were big enough for pelleting by centrifugation. Pellets contained the viral particles and were resuspended in a small volume of growth medium. Infecting murine cells with the concentrated vector lead to a higher transduction rate compared to the unconcentrated virus or the diluted virus (Fig. 7).
In addition, we determined the decay curve of the viral particles. Incubation at 37°C leads to a strong decrease of the infection rate with a half time of approximately 6.5 hours (Fig. 8). This short half life provides an enhanced safety regarding work and handling of our viral vector.
Transduction efficiency of our viral vector in murine cells was optimized to over 85%!
Specificity and Safety of MuLV
An important aspect for the function of our system as well as for its safety is the specificity of the vector regarding infection of different kind of cells. Cells that do not have this specific receptor are not targeted by the vector (Fig. 9; Fig. 10). To test the specificity of the system, different kinds of cells were incubated with the vector containing EGFP. EGFP is stably integrated by the system. We tested human cell lines, human embryonic kidney cells as well as human lung epithel carcinoma cells and breast cancer cells, for their susceptibility of being infected by the vector. In none of them, green fluorescent cells were observed (Fig. 10). Murine cells (NIH3T3) that express the mouse CAT-1 receptor were tested as a positive control (Fig. 10). Quantification by flow cytometry indeed showed that human cells were not transduced, whereas a large fraction of the mouse control cells were transduced with the same virus batch (Fig. 11, Fig. 12). 9E-G).

Figure 10: Infection of different cell lines with the viral vector derived from the murine leukemia virus.
Cells that were infected by viral particles express EGFP. Labjournal

Fig. 11: FACS analysis of murine cells (NIH3T3) incubated with the mCAT-1 specific viral vector
Murine cells were transduced with MuLV EGFP and analysed with flow cytometry after 48 hours of incubation.
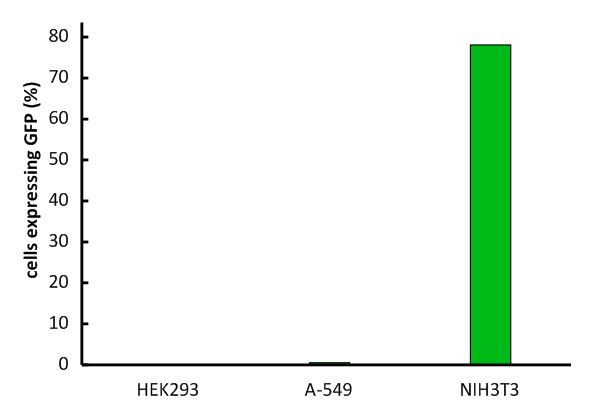
Fig. 12: Infection of different cell lines with the viral vector derived from the murine leukemia virus.
In order to investigate the specificity of the viral vector, two human cell lines, human embryonic kidney cells as well as lung epithel carcinoma cells, were infected and the percentage of cells expressing EGFP was analyzed by flow cytometry. Labjournal
The vector derived from the murine leukemia virus is specific for the murine CAT-1 receptor. Therefore it is not able to infect human cell lines making handling easy and safe!
 "
"


