Classification
Automatic Classification
function [mask seg] = automaticseeds(maskedImg)
end
Smoothness Index
For position prediction in virtual environments, jitter or noise in the output signal is not wanted while often present.
Since discovering smooth areas is a similar problem to jitter detection, a simple method for determining jitter can be used to measure non-jitter, smoothness [1].
It is assumed that jitter-free areas of a position signal do not differ in velocity.
Smooth areas don't differ in intensity, and therefore only low changes in velocity (intensity change) can be recorded.
For the reduction of noise, this operation is performed on the smoothed input image.
Then the smoothness $s$ of a pixel $p$ can be determined as:
\begin{equation}
s(p) = \sum\limits_{p' \in \mathcal{N}_k} \nabla(p') / \arg\max\limits_{p} s(p)
\end{equation}
Using a thresholding, $TS_l \leq s(p) \leq TS_u \wedge TI_l \leq I \leq TI_u$, different areas, such as background or pathogene, can be selected.
For the empirical choice of thresholds it can be argued that these are hand tailored to the specific case.
While this surely is true to a certain extent, the here presented method has been successfully tested on images from a completely different domain, and no changes to the thresholds have been made to make it work.
A proper theoretical evaluation is emphasized, however probably is not the aim of the iGEM competition.
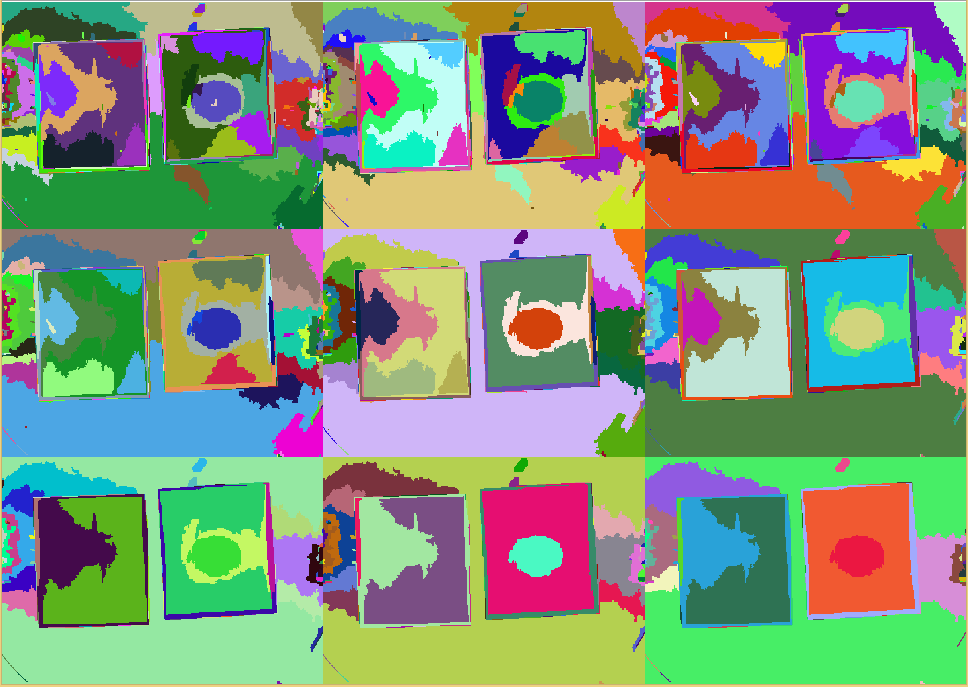
Finally selecting for the red region, this delivers the location of possible pathogenes.
Since the size of the agar chips is variable but fixed, a quantitative analysis can be performed by counting pixels for instance.
[1] Joppich M, Rausch D, Kuhlen T. Adaptive human motion prediction using multiple model approaches. In: Virtuelle und Erweiterte Realität, 10. Workshop der GI-Fachgruppe VR/AR. Shaker Verlag; 2013. p. 169–80.
Empirical Evaluation
Using our Matlab code we found the lower threshold for the smoothness index to be $TS_l = 0.85$ and the upper threshold $TS_u = \infty$.
Similarly for $TI_l = 235$ and $TI_u = \infty$.
For these settings we can find a response already in images taken after 42 minutes.
Ideally one would rate the quality of the image segmentation using some ground truth, such as manual delineations. This yet has to be done for our method.
However, from visual observations, our method is showing promising results.
|
 "
"