Team:ETH Zurich/expresults/qs
From 2014.igem.org
Quorum Sensing
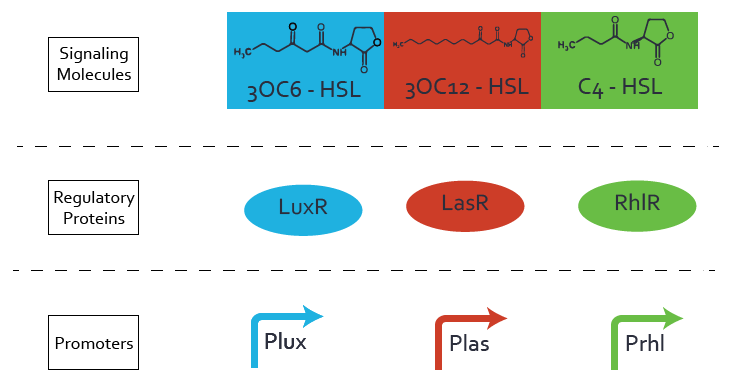
For our Mosiacoli project, we were looking for molecular systems that allow orthogonal cell-to-cell communication in order to implement connected XOR logic gates. We decided to exploit the quorum sensing systems LuxI/LuxR, LasI/LasR, and RhlI/RhlR in order to achieve the required orthogonal cell-to-cell communication. We developed a model for these cellular information processing. Even though the corresponding inducer molecules are commercially available and the systems often used, in particular in iGEM projects (e.g. [http://parts.igem.org/Part:BBa_R0062 pLux (BBa_R0062)], '[http://parts.igem.org/Frequently_Used_Parts Top 10 Most used promoters]' with 246 uses), potential crosstalk activity between the different systems may be a severe problem (e. g. Tokyo_Tech 2013, Peking University 2011).
In order to address this challenge, we measured a) a given promoter with its corresponding regulator and a different inducer molecule, b) a given promoter with an unspecific regulator and a particular inducer, c) a given promoter with both regulator and inducer being unspecific, and always included the correct combination of inducer molecule, regulator and promoter as a positive control. This gives in total 27 possible combinations. The output was assessed via sfGFP and measured in terms of fluorescence on microtiter-plate scale.
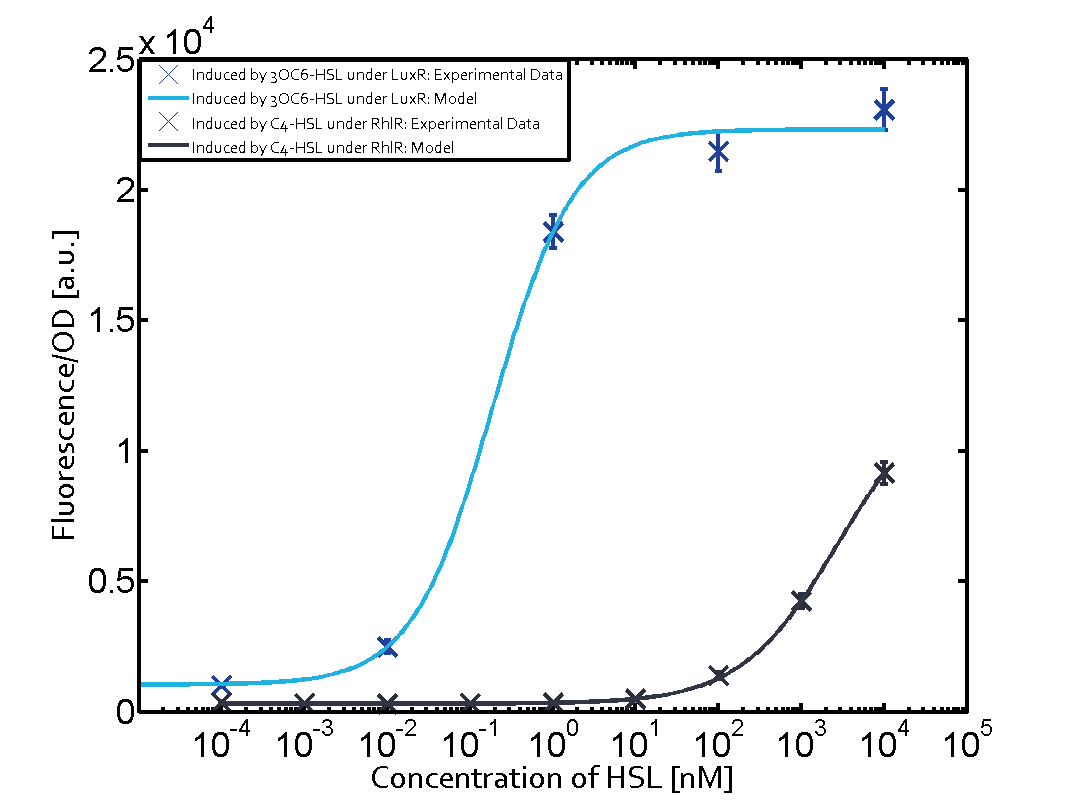
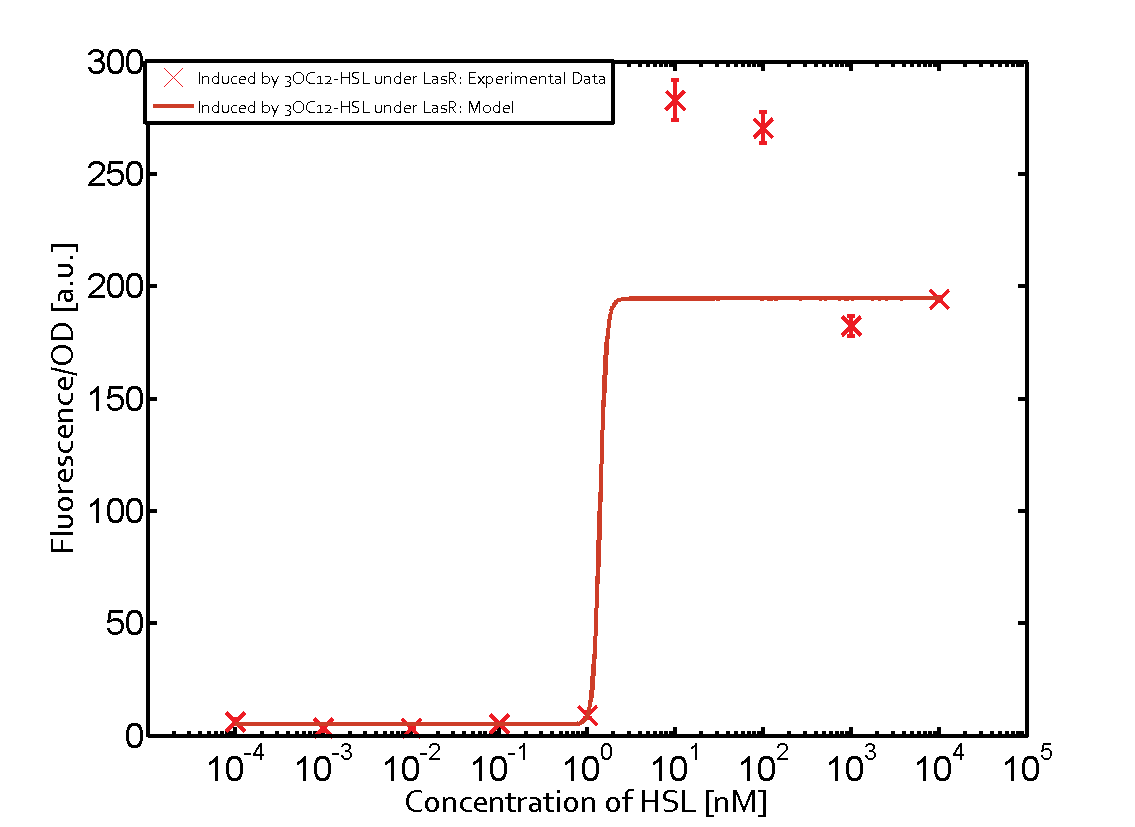
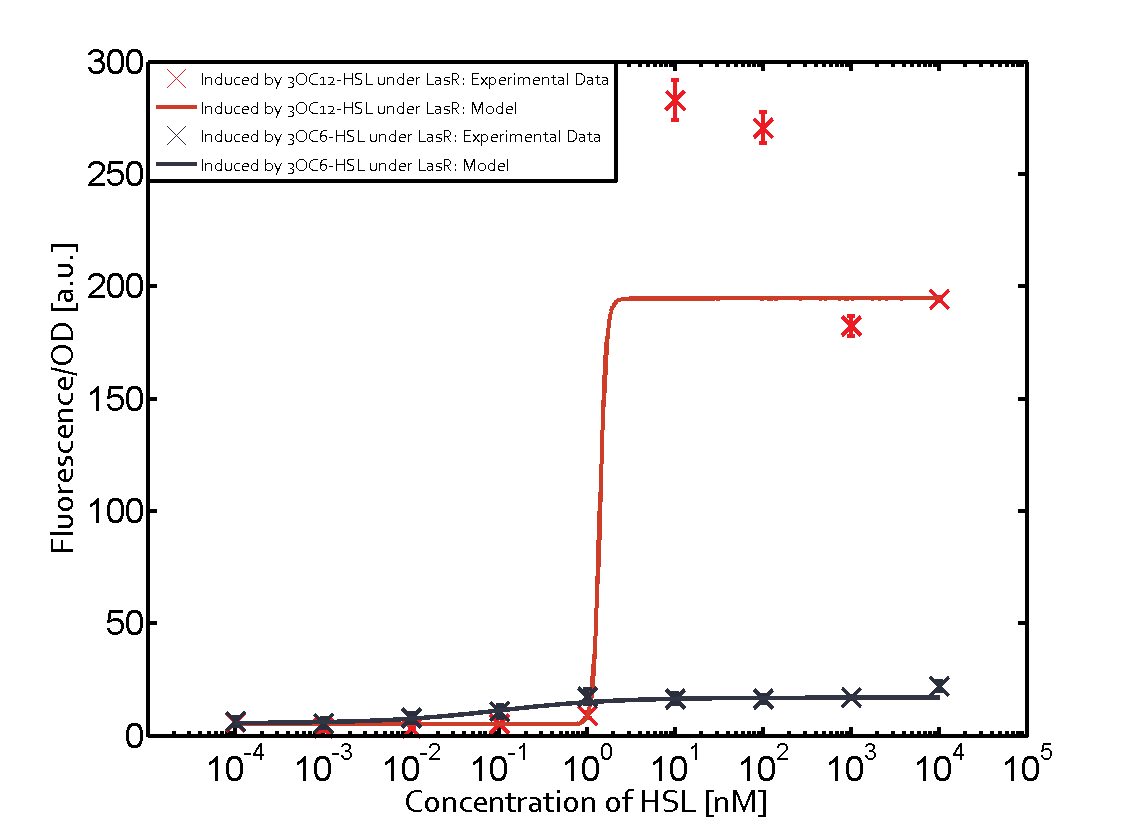
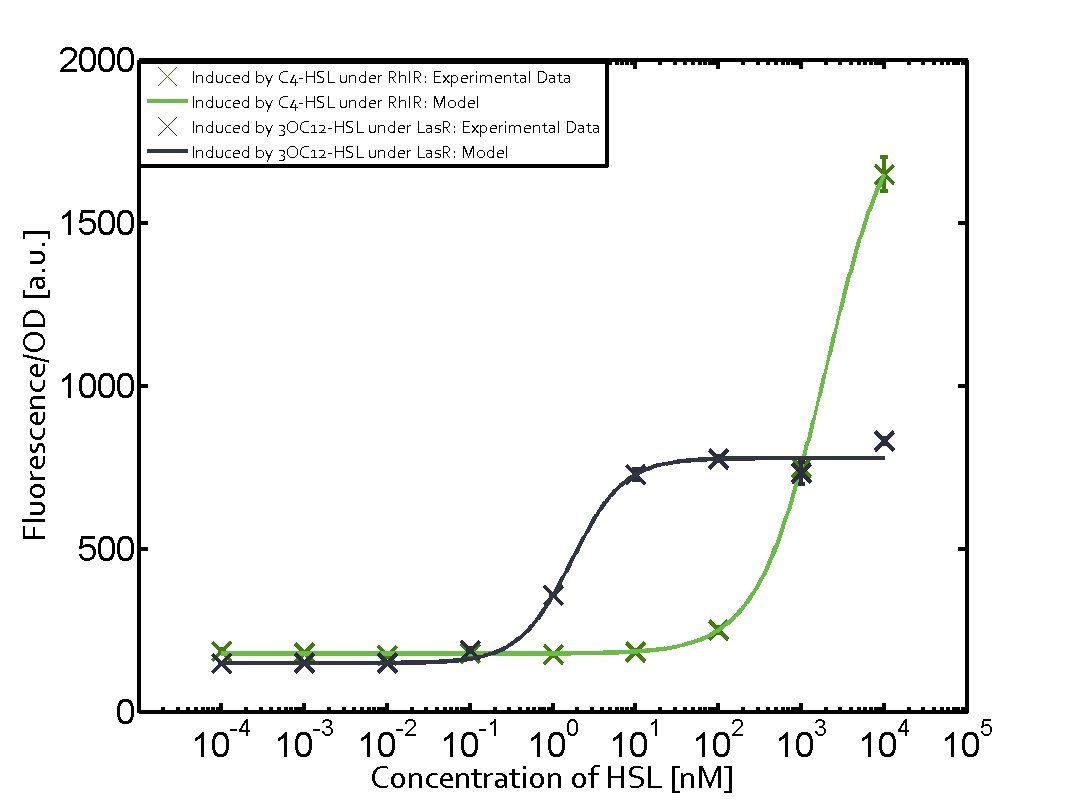
Summary of experimental results regarding quorum sensing
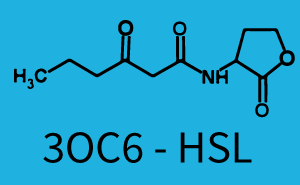
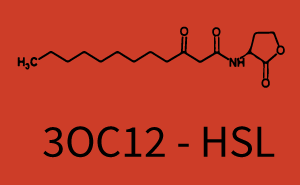
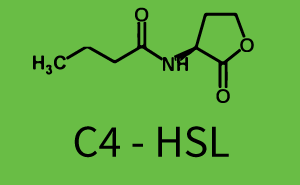
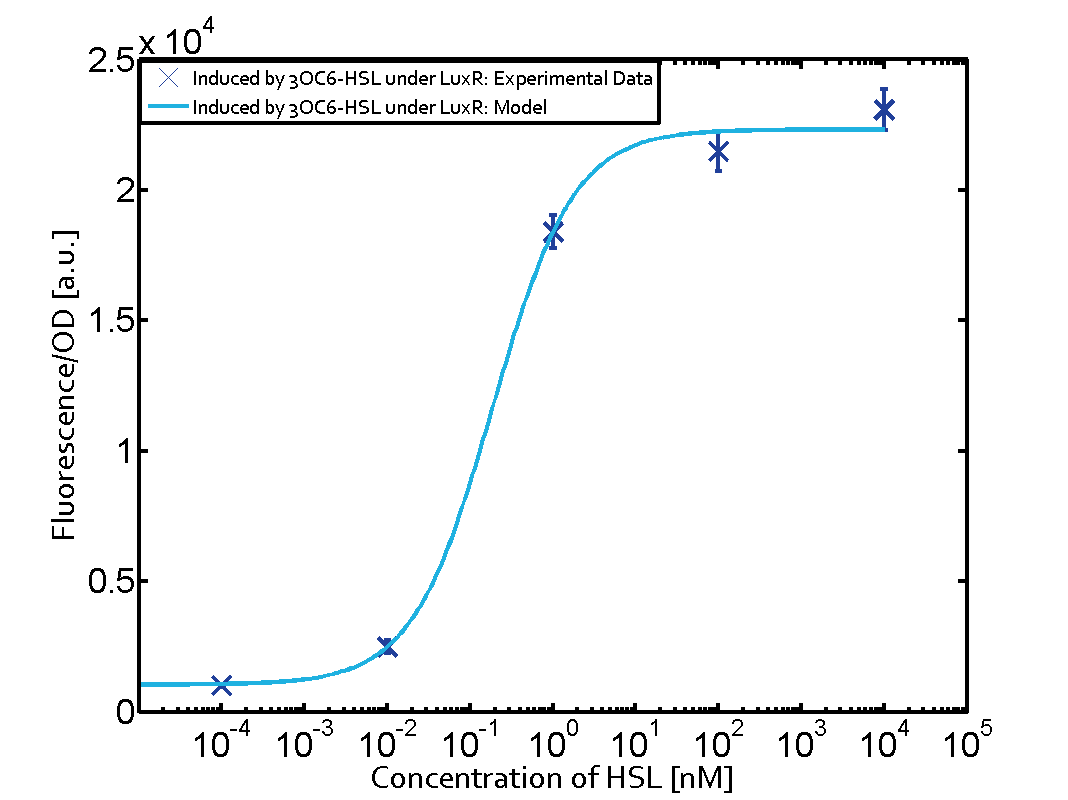
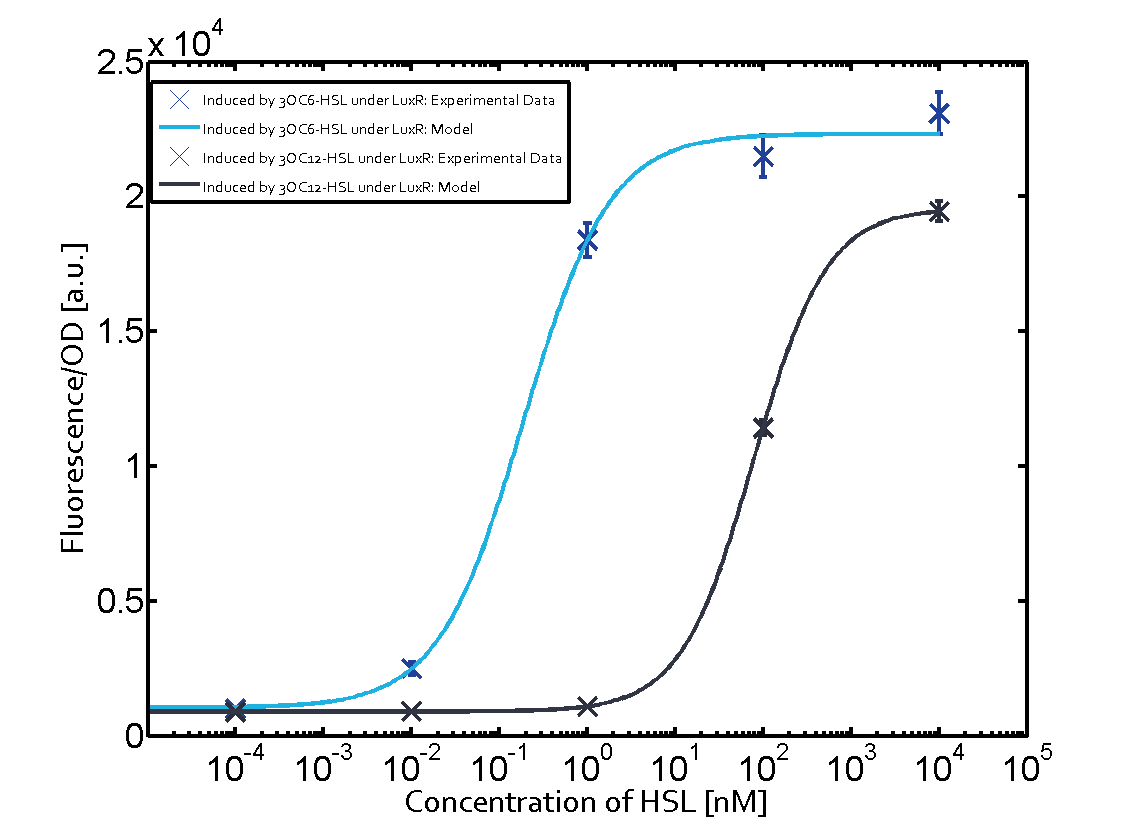
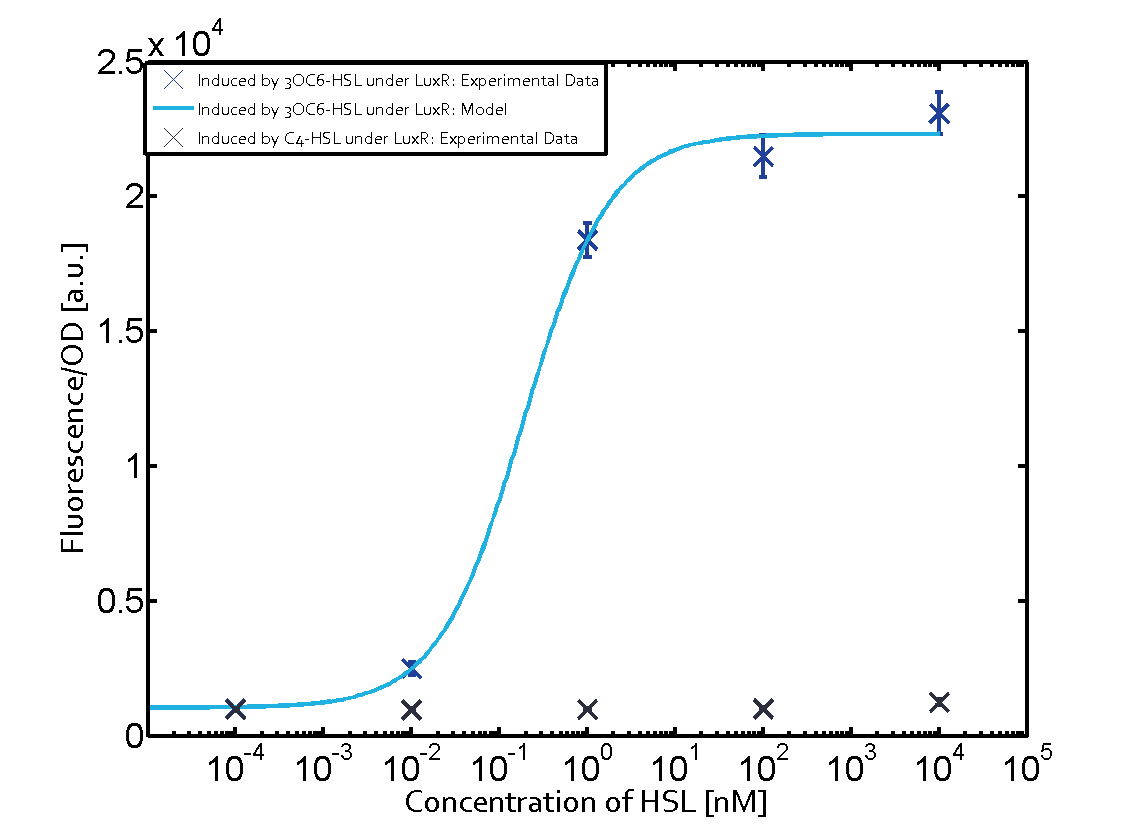
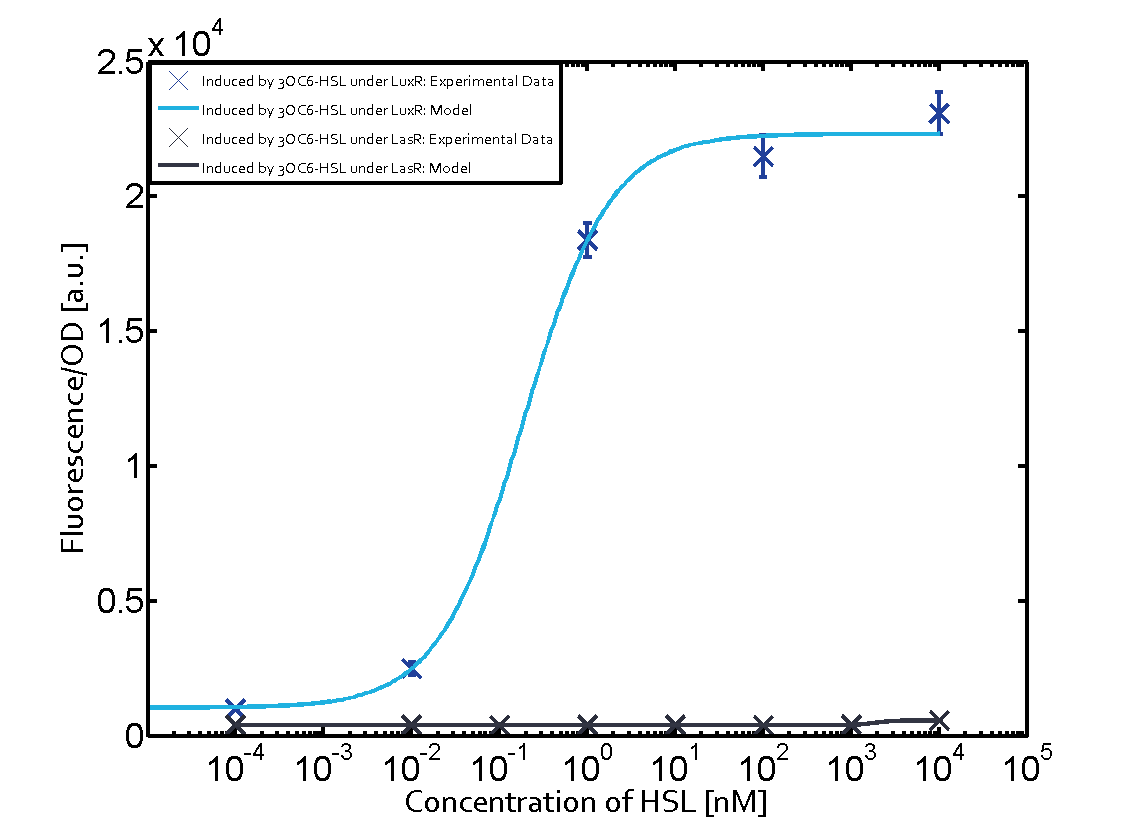
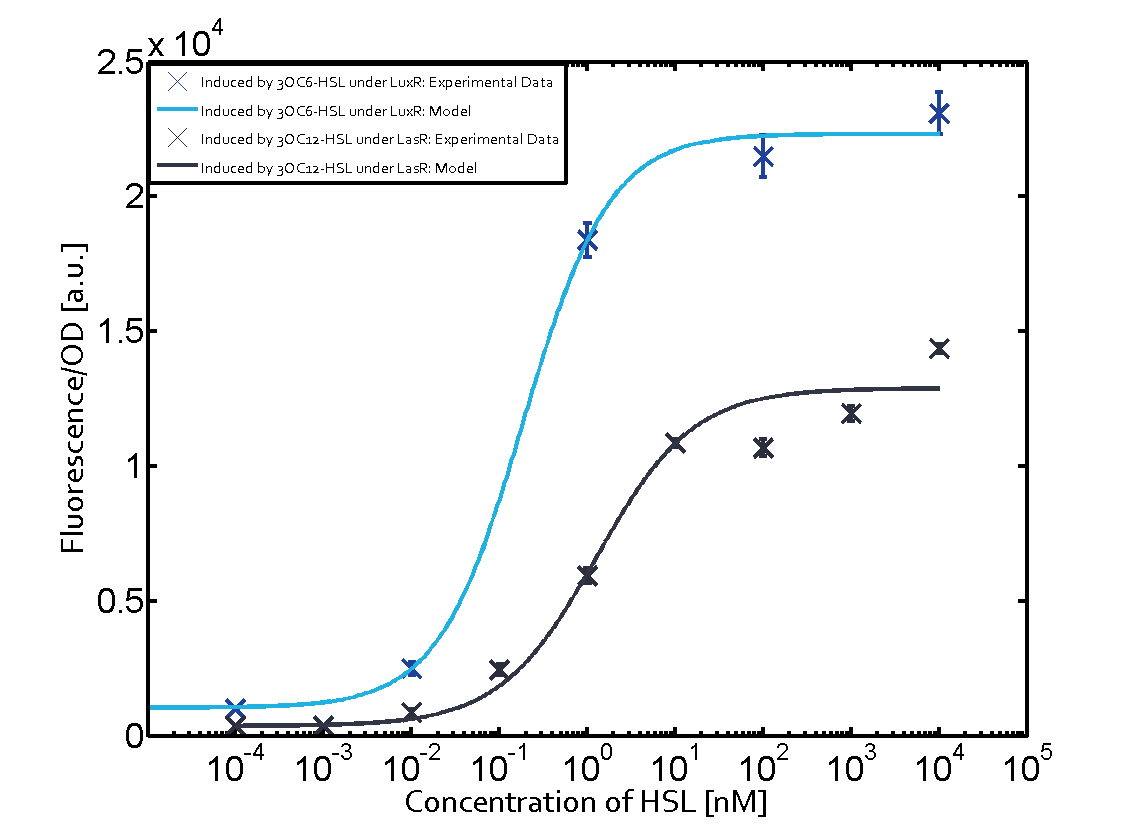
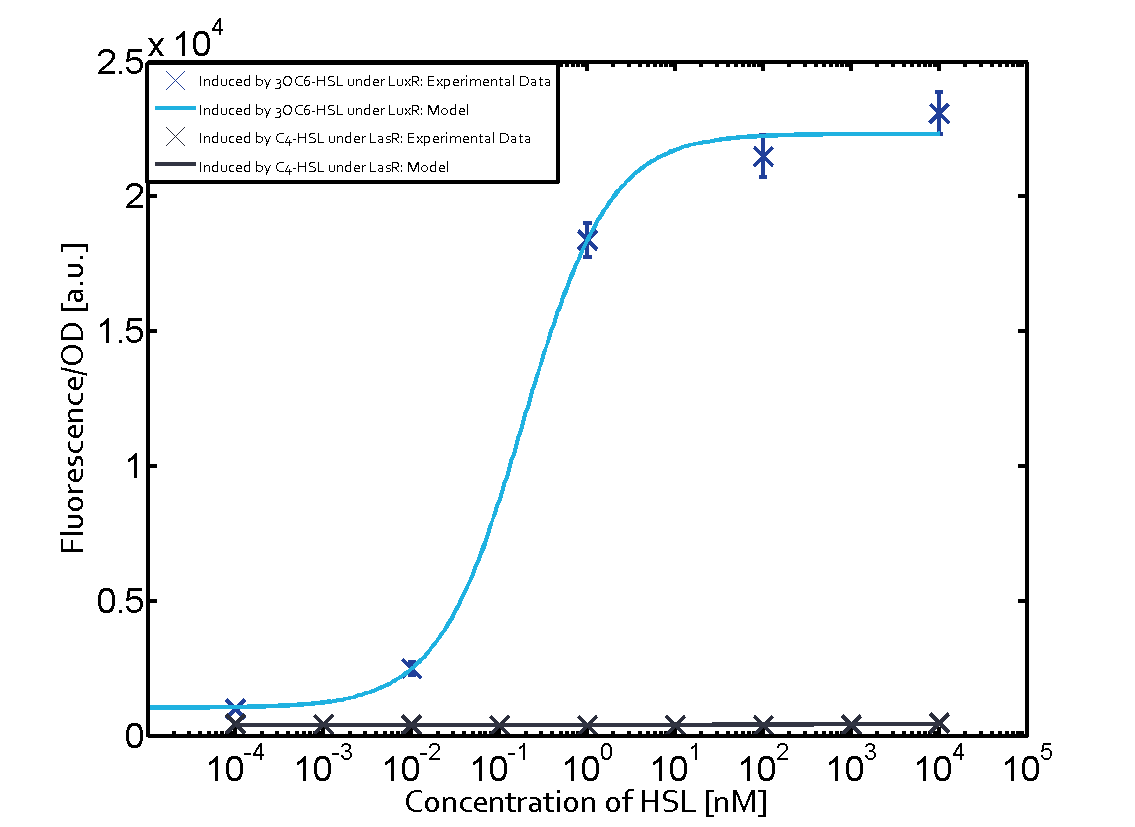
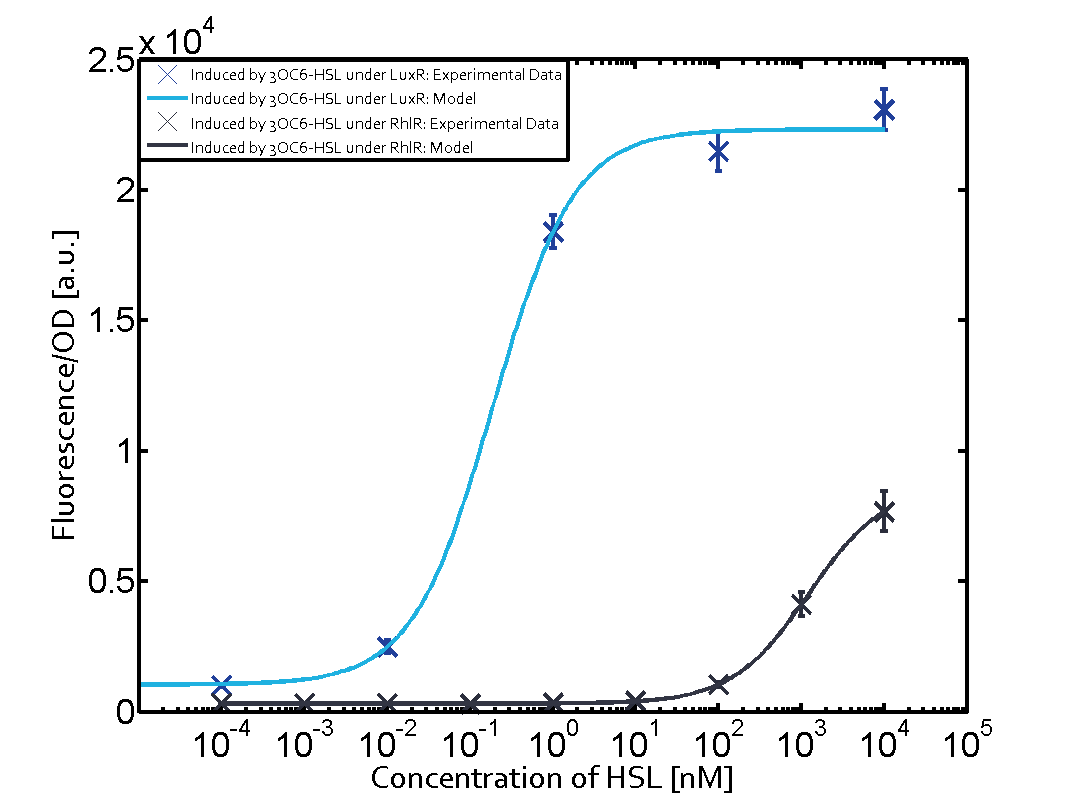
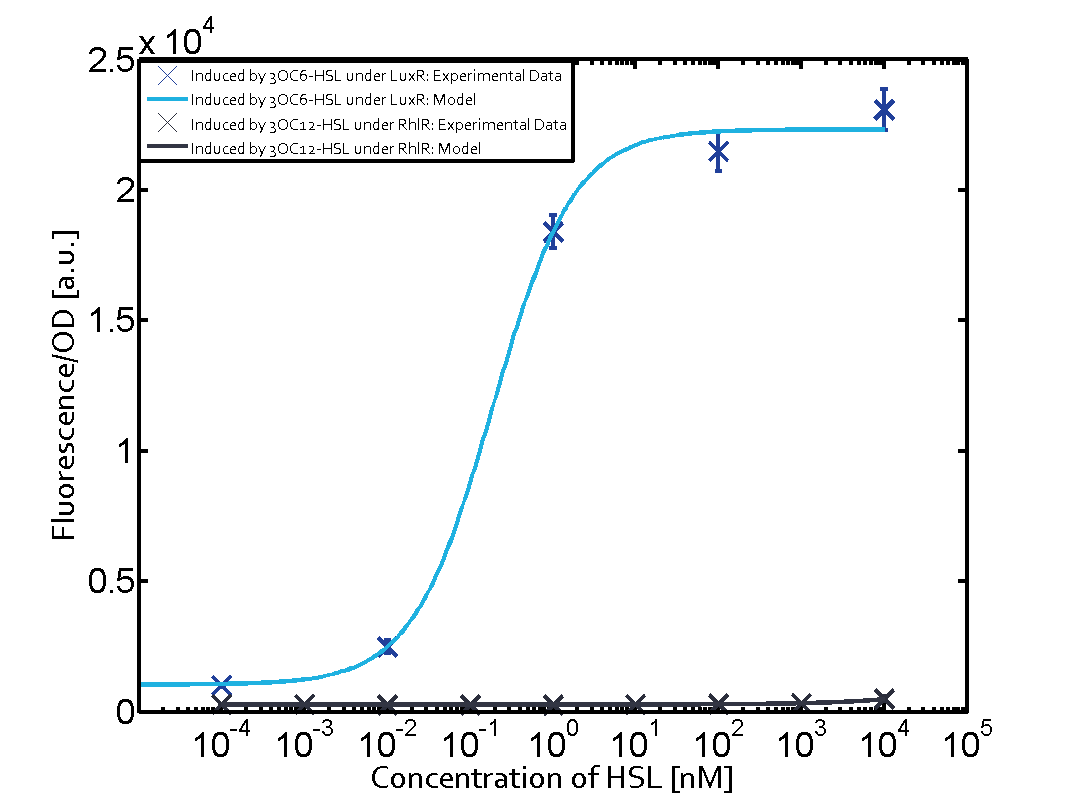
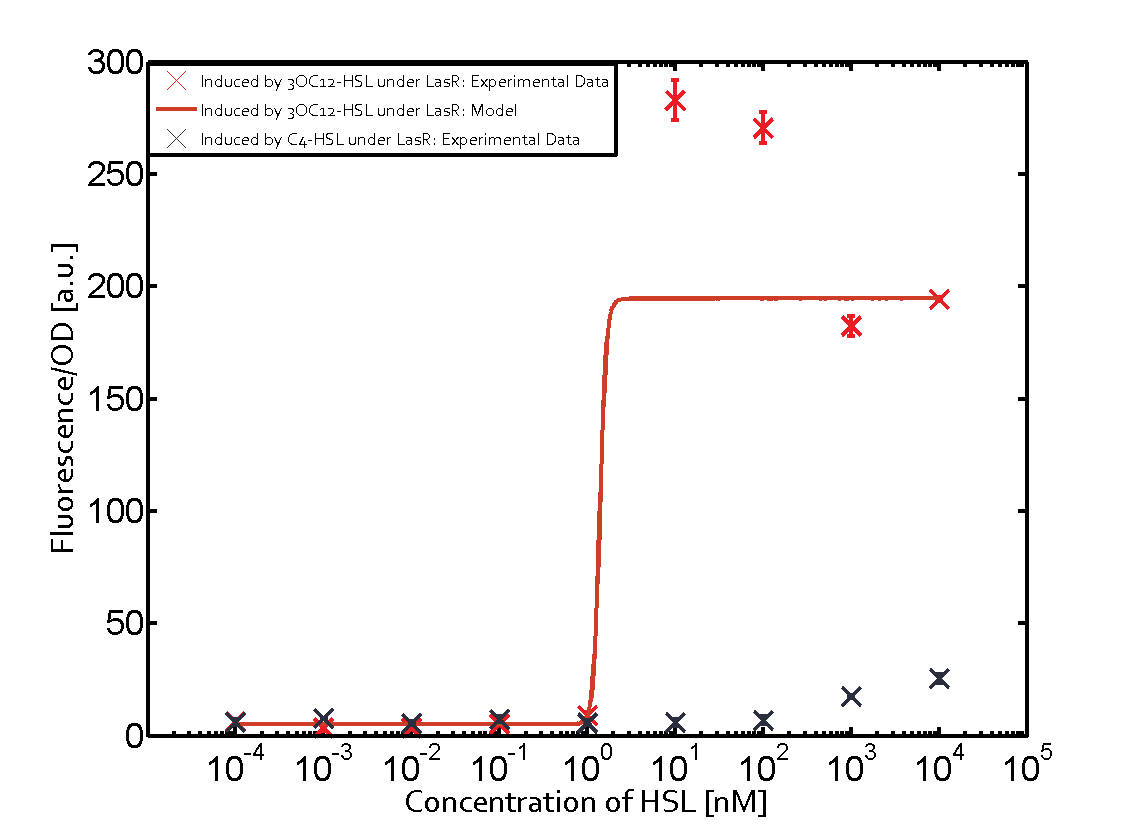
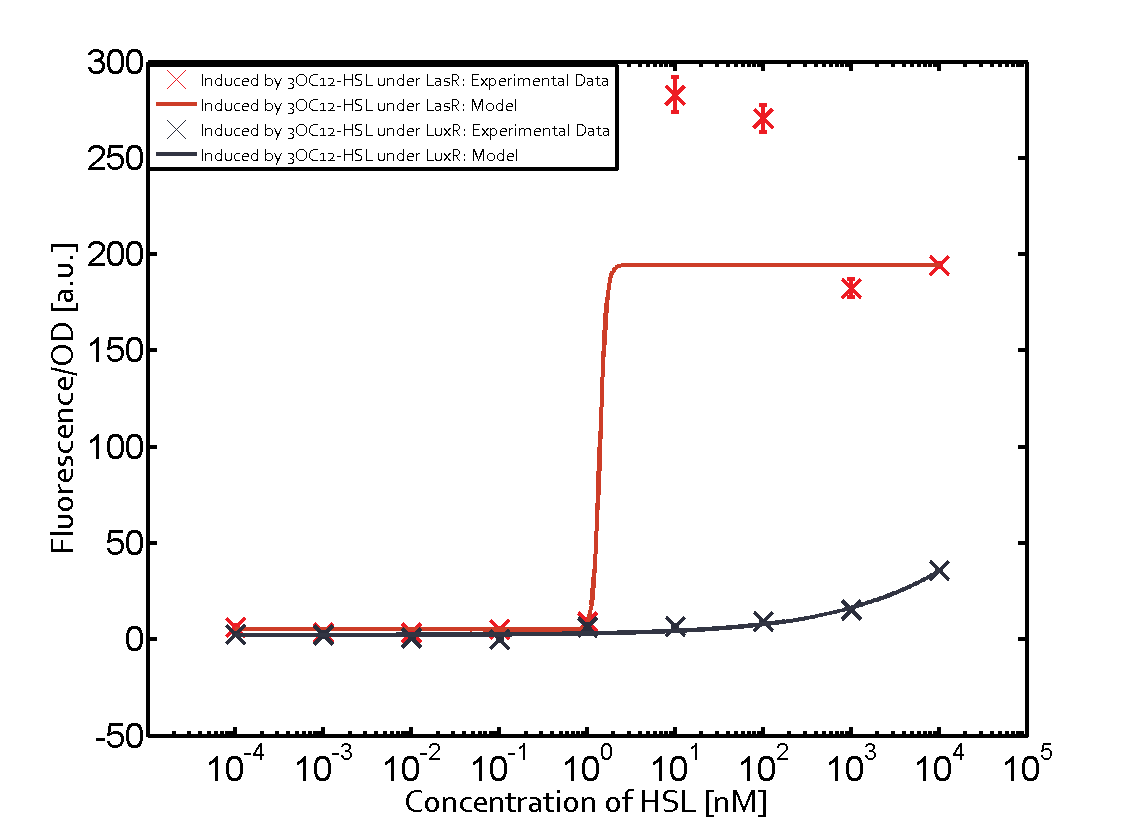
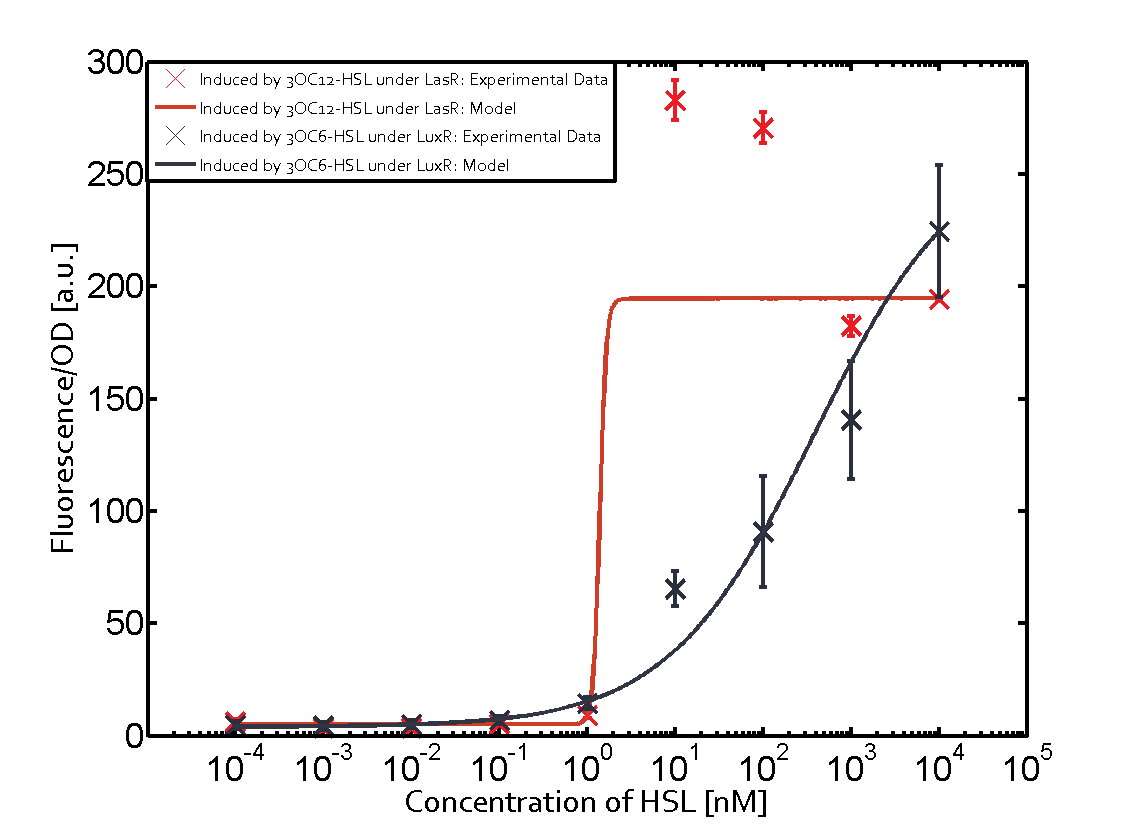
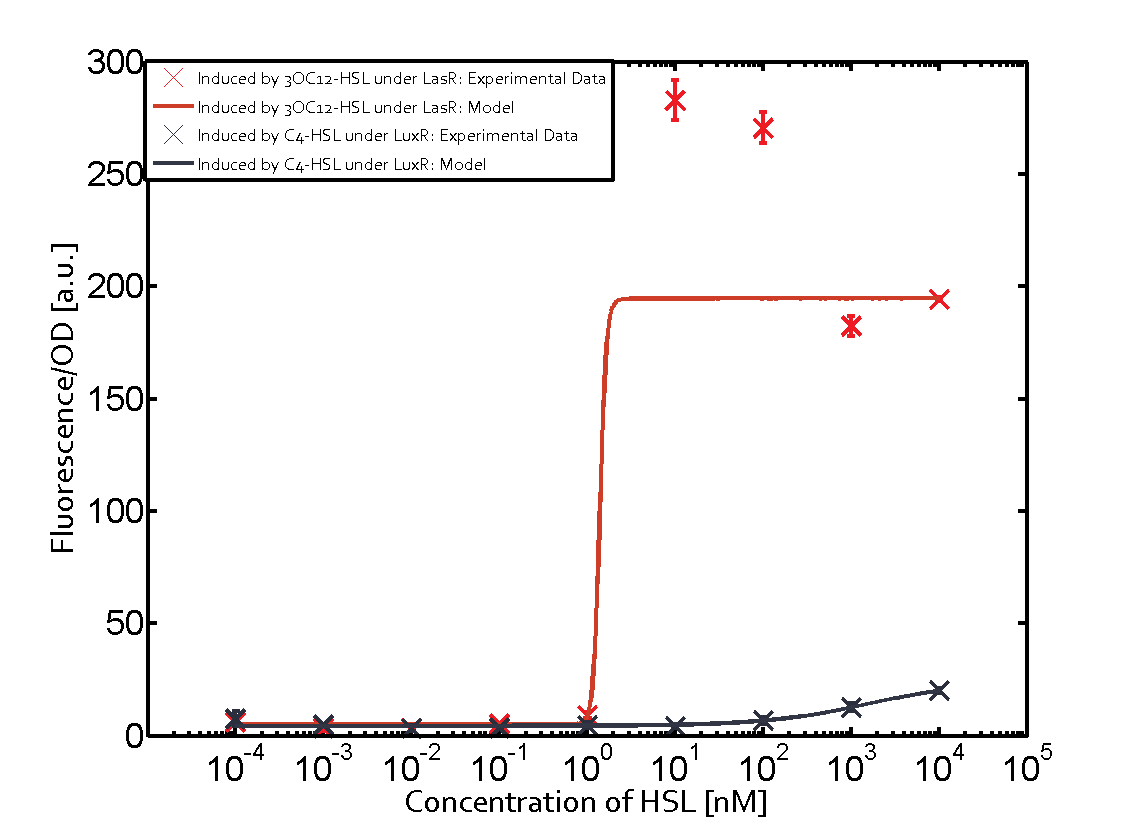
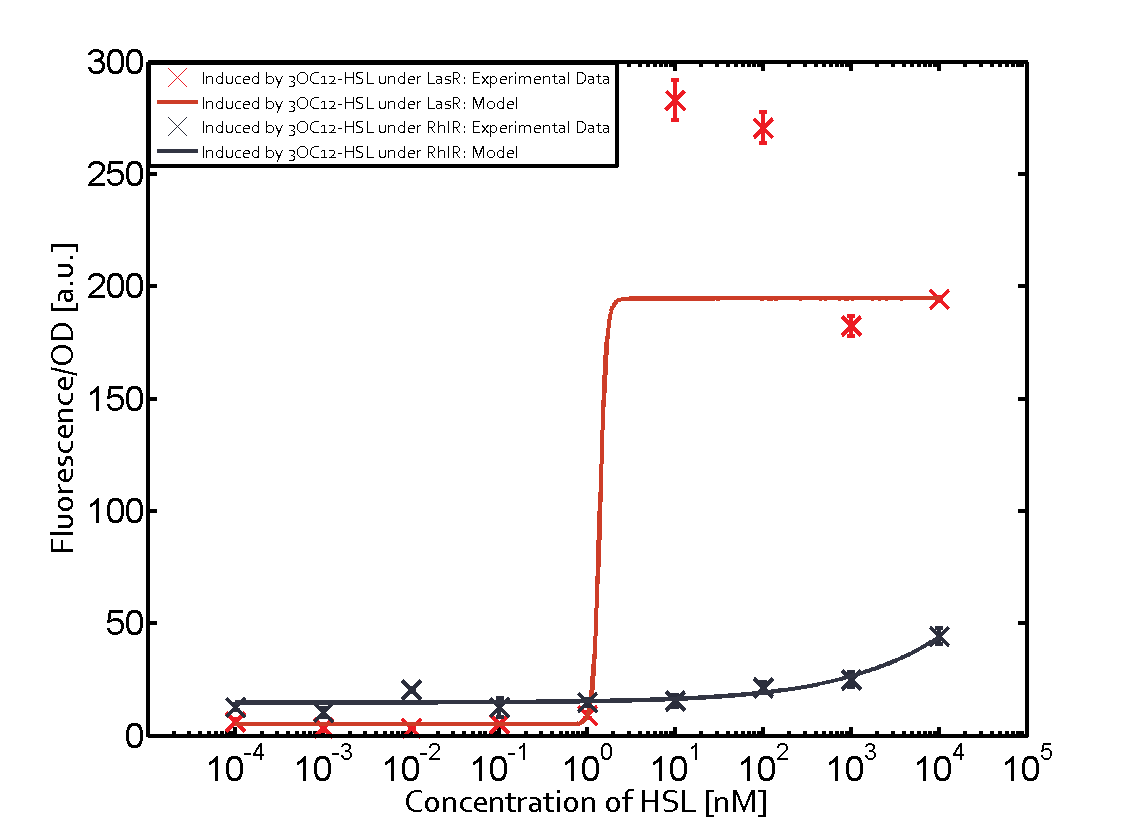
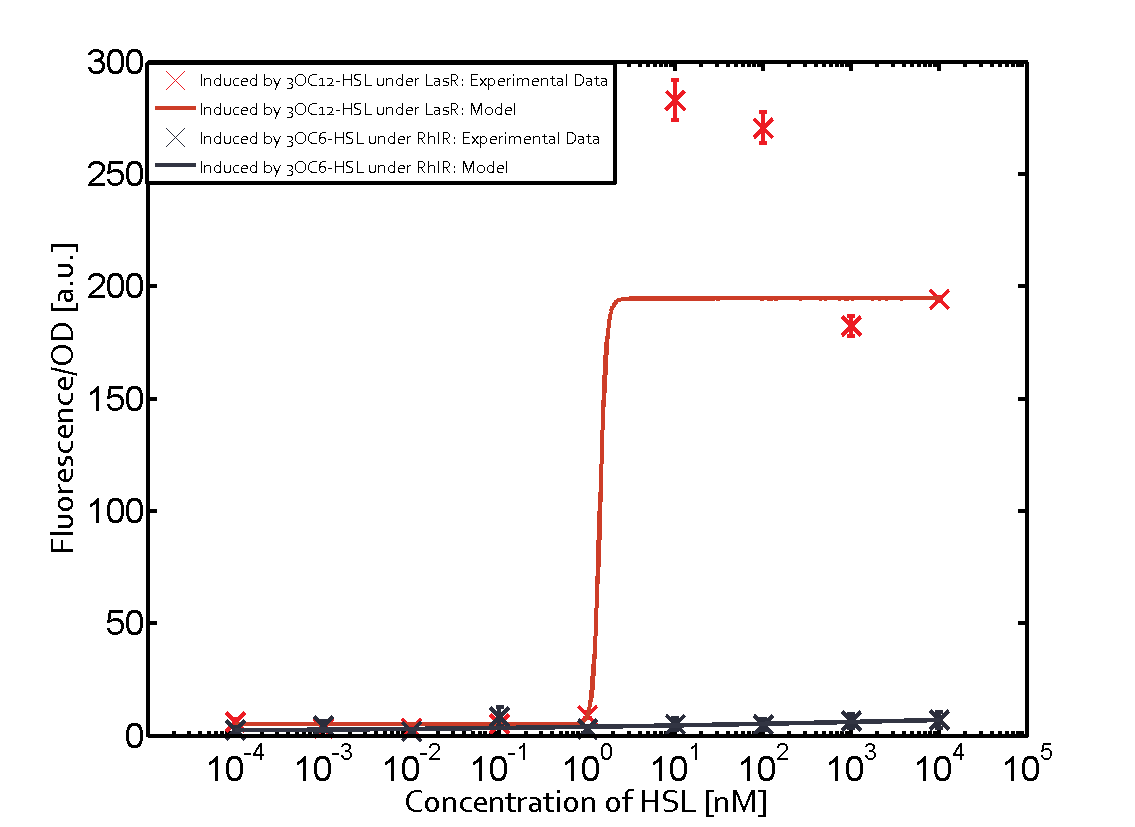
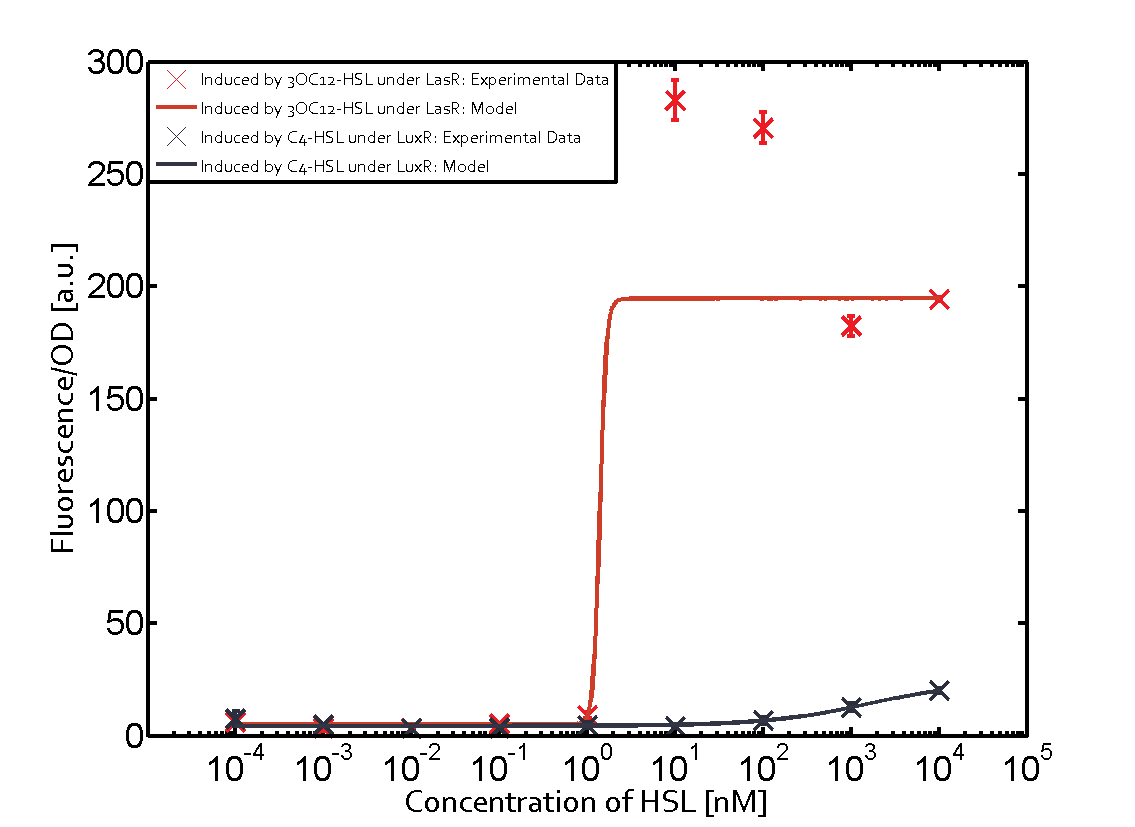
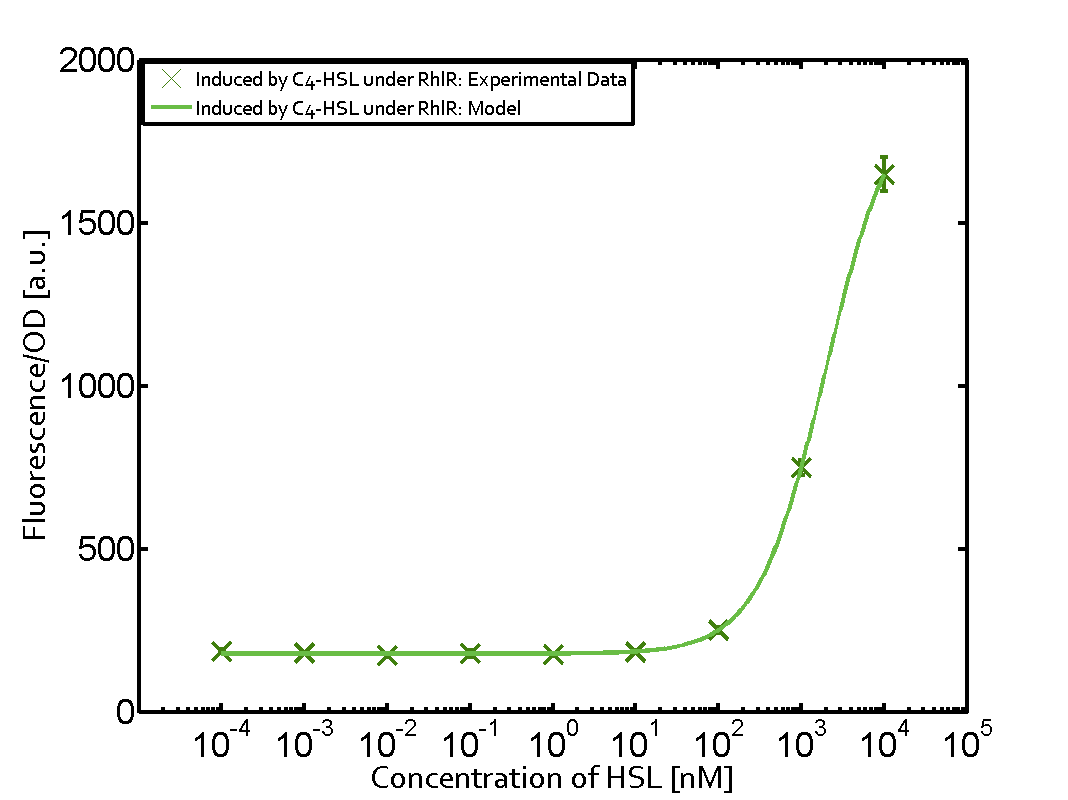
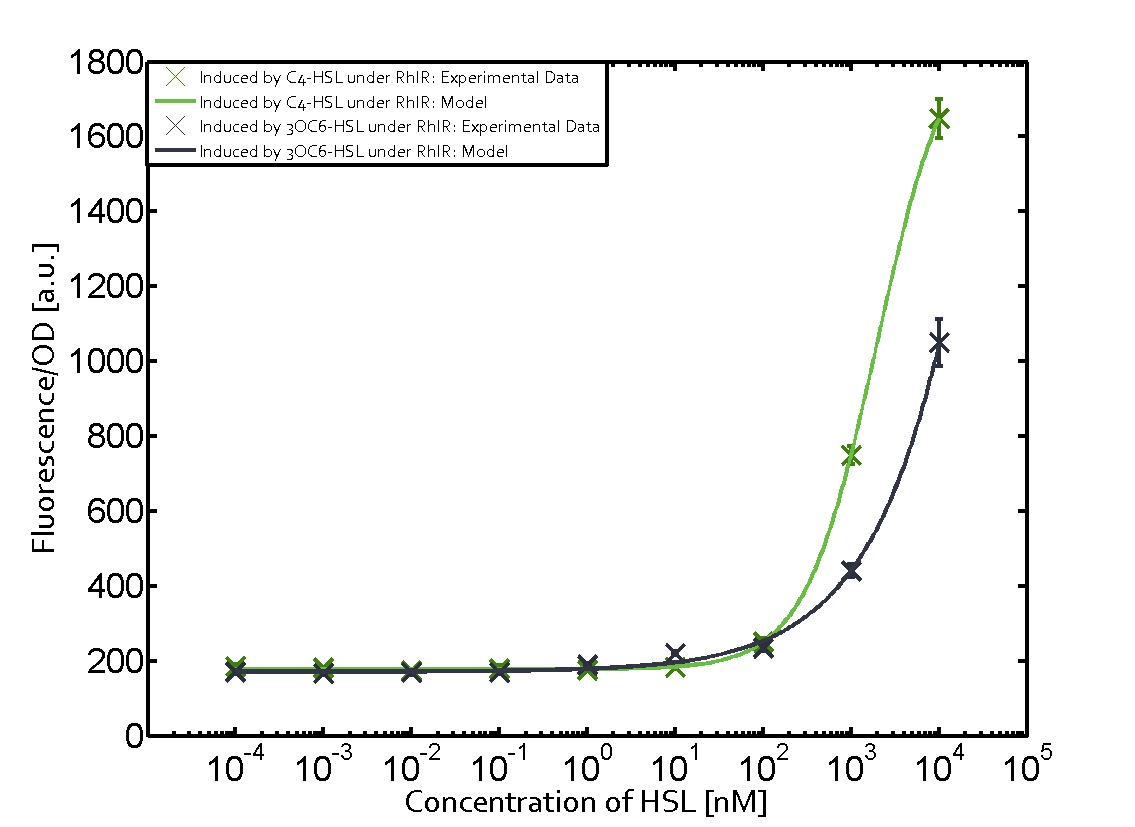
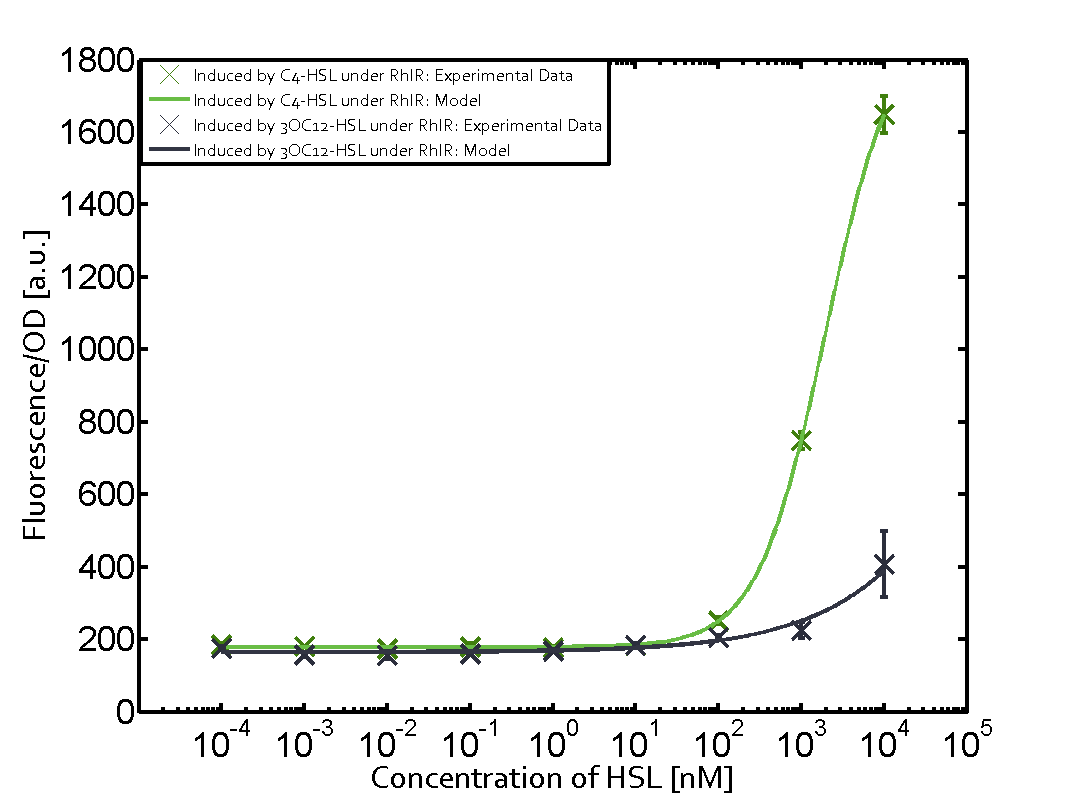
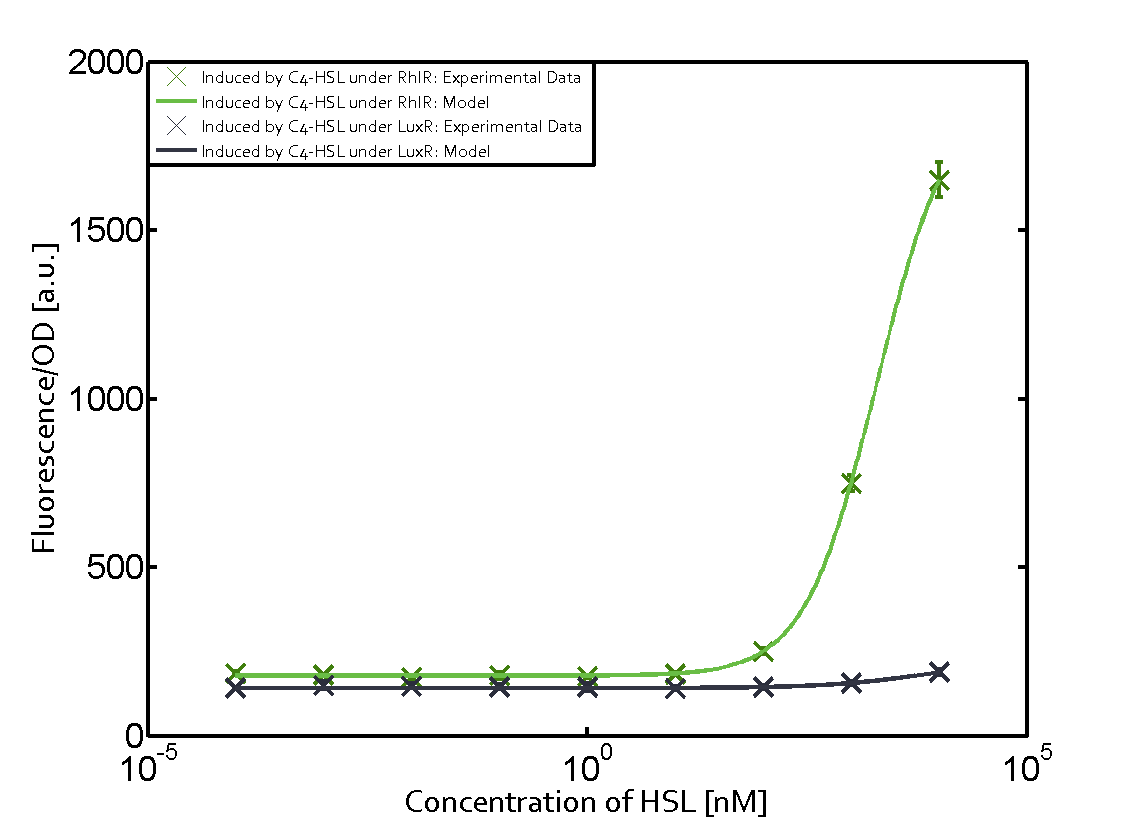
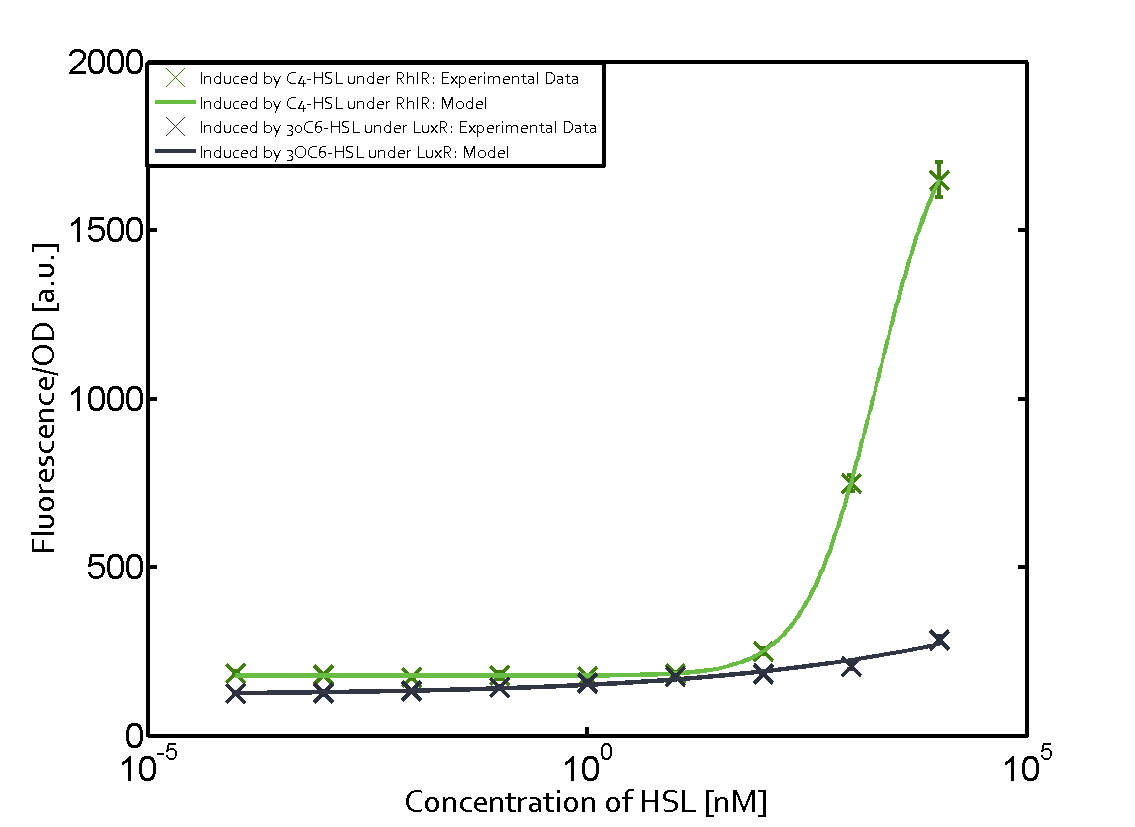
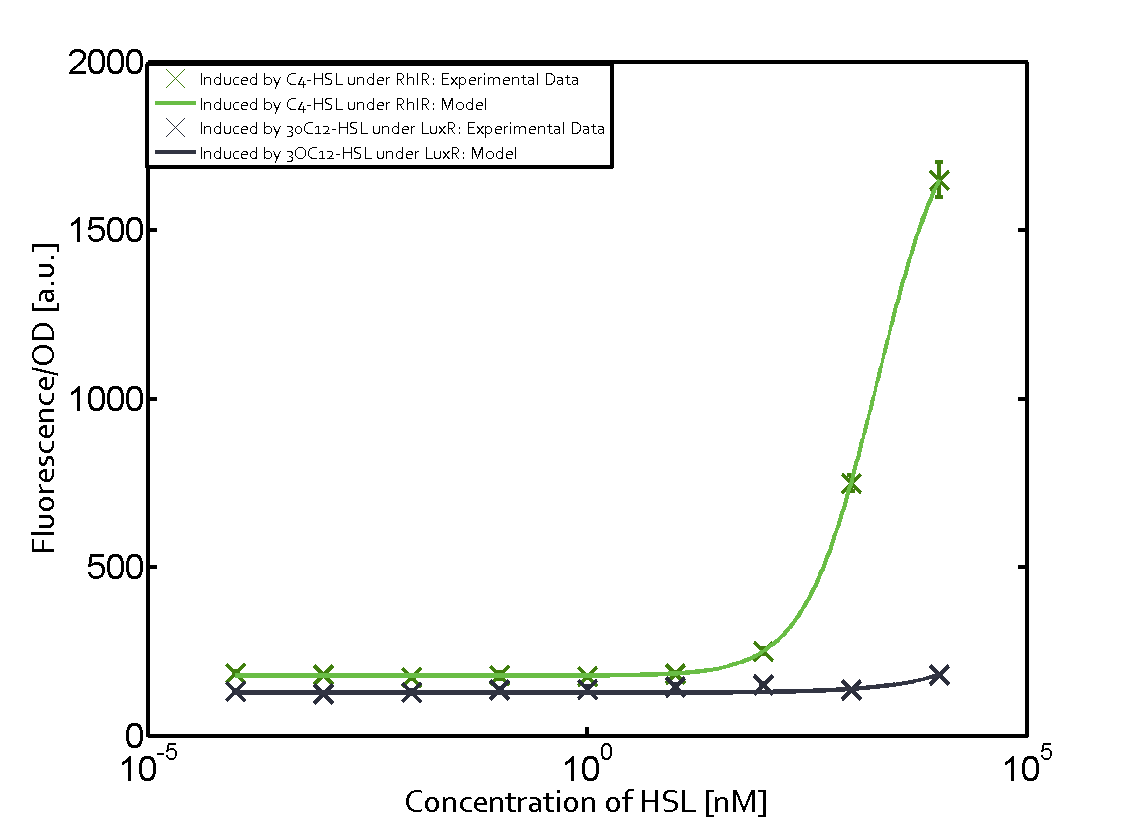
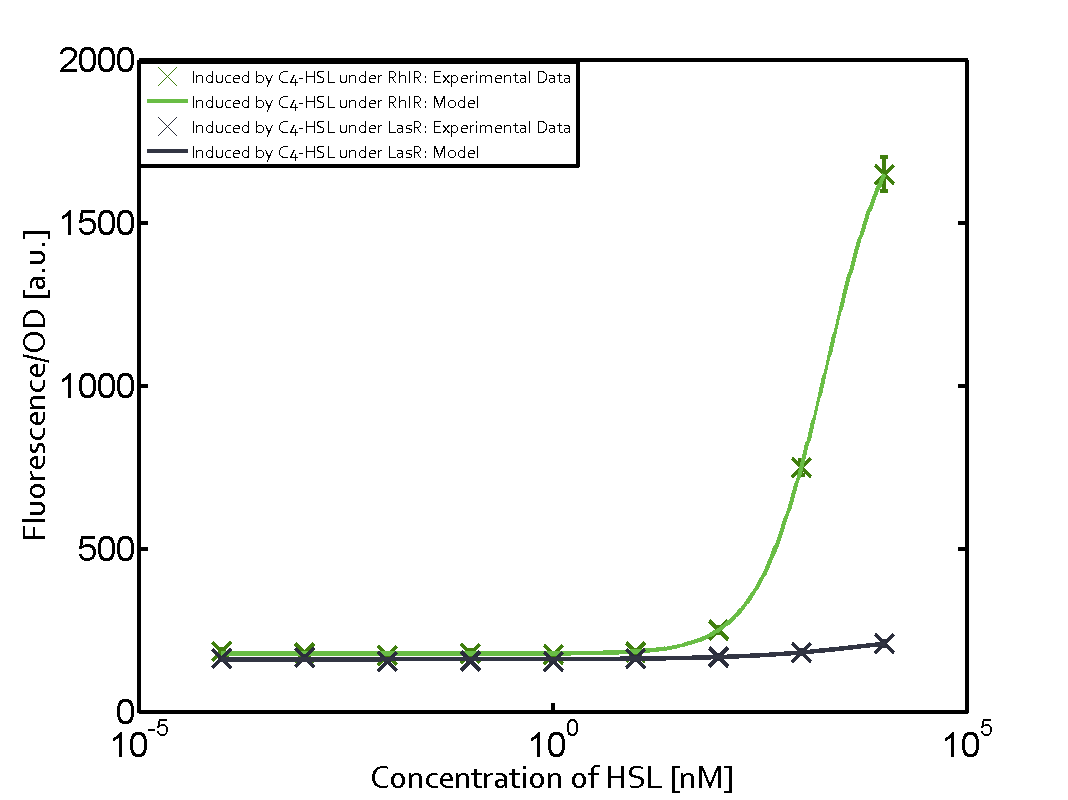
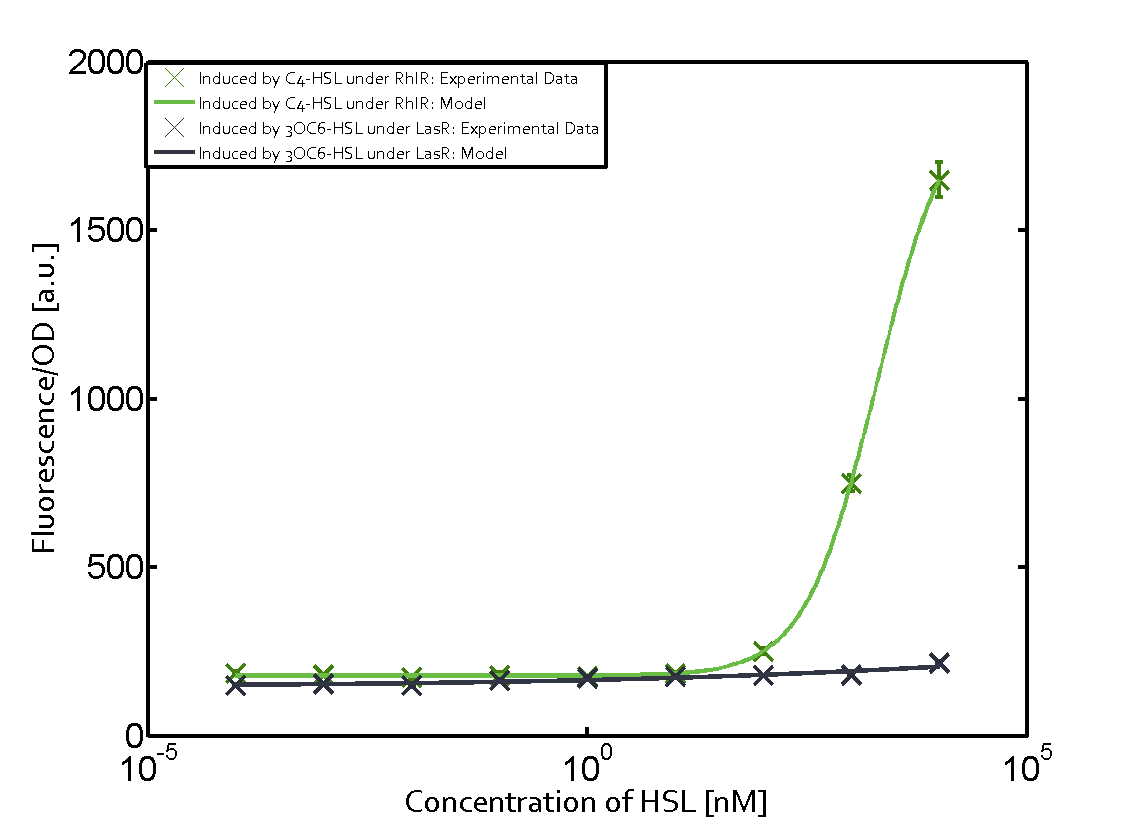
The following matrices serve as an overview summarizing the most significant results of our experiments to characterize crosstalk on different levels. On the horizontal top row we see the three different inducer molecules (3OC12-HSL, 3OC6-HSL, C4-HSL). In the top left corner we see the quorum sensing promoter used for all the experiments summarized in this matrix. On the vertical axis we see the three regulators ( LuxR, LasR, RhlR). These matrices are giving an overview of the experimental results conducted in relation with quorum sensing and crosstalk. The graph shown in each matrix on the very top left describes the situation where the correct autoinducer molecule has bound the corresponding regulator and this complex has then induced the correct promoter. The solid lines in the graphs show the model data, whereas the data points indicated with standard deviation show experimental data in triplicates (mean values of triplicate micro titerplate measurements).
Conclusion of crosstalk experiments
As shown in the graphs in the matrices above, we found and quantitatively characterized all three levels of crosstalk. The three levels were the following:
- A given promoter with its corresponding regulator and a different inducer molecule
- A given promoter with an unspecific regulator and a particular inducer
- A given promoter with both regulator and inducer being unspecific
Unspecific inducers binding to the regulators as well as unspecific binding of the regulator to another promoter species was observed in almost all possible combinations. To conclude, we were not able to find an orthogonal quorum sensing pair out of the three systems investigated (LuxI/LuxR, LasI/LasR, or RhlI/RhlR). While we see a significant effect when implementing the influence of these crosstalks (on an inducer-, regulator- and promoter-level) in our whole cell model, the logic gate still continues to function for a range of inputs at physiological concentrations.
 "
"