Team:SF Bay Area DIYbio/Parts
From 2014.igem.org
| |||||||||||||
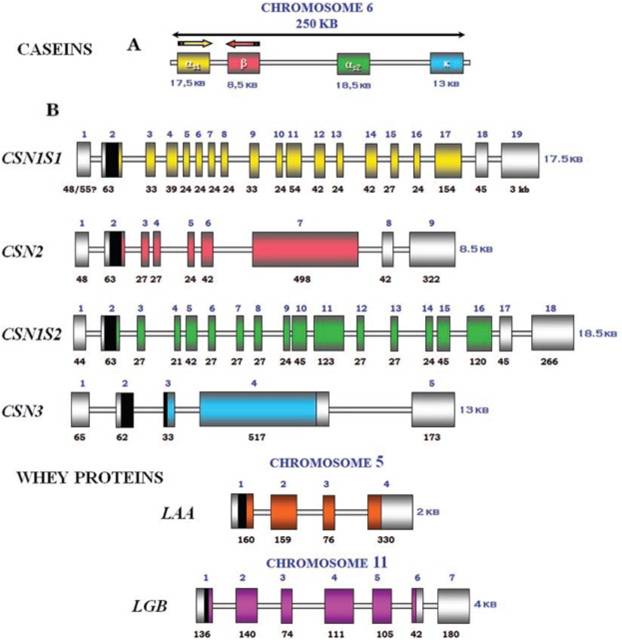
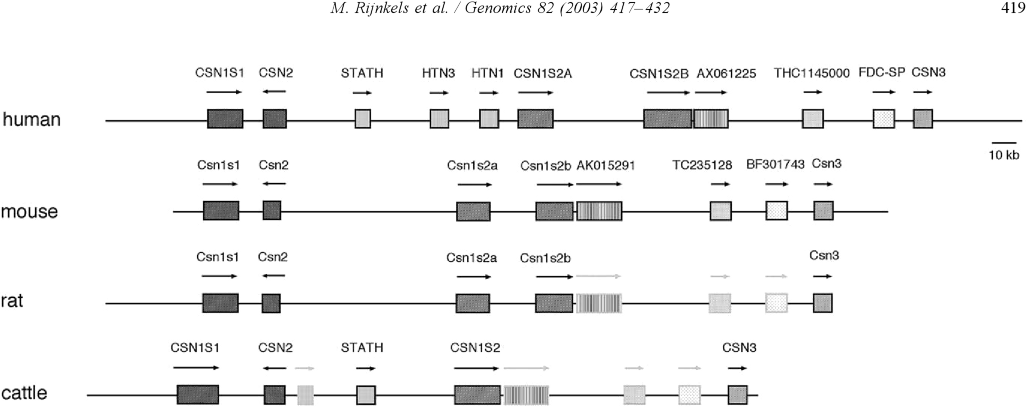
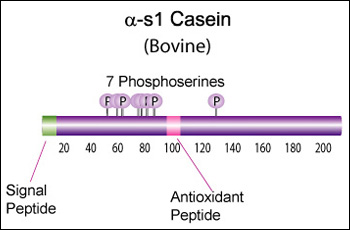
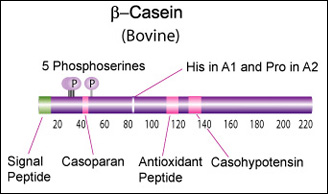
IntroductionCheese made from cow's milk contains four major cheese proteins: alpha S1, alpha S2, beta and kappa casein, with alpha S1 and beta casein being the most abundant components. Many genetic variants of these four casein genes have been identified in different breeds of cows[http://www.ncbi.nlm.nih.gov/pubmed/19841193], often associated with various milk and cheese processing parameters (such as coagulation time)[http://www.ncbi.nlm.nih.gov/pubmed/23746587], or health effects. Human breast milk is somewhat different in composition - humans do not have a functioning alpha S2 casein gene, and the dominant proteins are beta and kappa casein, rather than alpha S1 and beta. Interestingly, because of intensive research by the dairy industry, far more is known about bovine casein genetic variants ant their consequences than in human. In addition to the casein genes, we will also express Fam20C, a Golgi kinase responsible for phosphorylating the caseins Exon-intron structure of bovine milk proteins:Organization of the casein genes in the human, mouse, rat, and cattle genome:Alpha-s1 casein (CSN1S1)Major component of cow's milk, along with beta casein. α-s1 Casein is the most prevalent form of casein in bovine milk. It has been reported to exhibit antioxidant and radical scavenging properties.1 It has also been reported to be involved in the transport of and casein from the endoplasmic reticulum to the Golgi apparatus. [Chanat, E., J. Cell Sci., 112, 3399-3412 (1999)] [http://www.sigmaaldrich.com/life-science/metabolomics/enzyme-explorer/enzyme-reagents/casein.html] Genetic variants & health effectsA recent review of milk protein nomenclature (Farrell et al., 2004) indicated 8 αs1-CN (A, B, C, D, E, F, G, H) [...] variants in the Bos genus. [http://www.ncbi.nlm.nih.gov/pubmed/19841193] CSN1S1 C positively affected milk coagulation [http://www.ncbi.nlm.nih.gov/pubmed/23746587] Sequences
Bovine alpha-S1-casein is a 214 AA protein, including a 15 AA secretion signal. Alpha-S1 is a key protein in milk protein allergy, and there is some data available on immunoglobulin E (IgE)-binding epitopes (i.e. antigenic peptides) in different genetic variants (Lisson et al., 2014), but there seems to be a lot of variability from patient to patient, and it is not clear (to me at least) what the health effects are of the different variants. Until we can figure out the health effects, I think we should focus on CSN1S1*C for its improved coagulation properties: >gi|195973860|gb|[http://www.ncbi.nlm.nih.gov/protein/ACG63494.1 ACG63494.1]| alpha S1 casein [Bos taurus] MKLLILTCLVAVALARPKHPIKHQGLPQEVLNENLLRFFVAPFPEVFGKEKVNELSKDIGSESTEDQAME DIKQMEAESISSSEEIVPNSVEQKHIQKDDVPSERYLGYLEQLLRLKKYKVPQLEIVPNSAEERLHSMKE GIHAQQKEPMIGVNQELAYFYPELFRQFYQLDAYPSGAWYYVPLGTQYTDAPSFSDIPNPIGSENSGKTT MPLW Human alpha-S1-casein is present at much lower concentration than in bovine milk, and is only 185 AA: >gi|1345671|sp|P47710.1|CASA1_HUMAN RecName: Full=Alpha-S1-casein MRLLILTCLVAVALARPKLPLRYPERLQNPSESSEPIPLESREEYMNGMNRQRNILREKQTDEIKDTRNE STQNCVVAEPEKMESSISSSSEEMSLSKCAEQFCRLNEYNQLQLQAAHAQEQIRRMNENSHVQVPFQQLN QLAAYPYAVWYYPQIMQYVPFPPFSDISNPTAHENYEKNNVMLQW Alignment between the bovine reference sequence (A variant), bovine C variant, and human alpha-S1-casein (only 33% sequence identity): P02662 1 MKLLILTCLVAVALARPKHPIKHQGLPQEVLNENLLRFFVAPFPEVFGKEKVNELS--------------KDIGSESTED 66 ACG63494 1 MKLLILTCLVAVALARPKHPIKHQGLPQEVLNENLLRFFVAPFPEVFGKEKVNELS--------------KDIGSESTED 66 P47710 1 MRLLILTCLVAVALARPKLPLRY---PERLQNPSESS---EPIPLESREEYMNGMNrqrnilrekqtdeiKDTRNESTQN 74 P02662 67 QAMEDIKQMEAESISSSEEIVPNSVEQKHIQKEDVPSERYLGYLEQLLRLKKYKVPQLEIVPNSAEERLHSMKEGIHAQQ 146 ACG63494 67 QAMEDIKQMEAESISSSEEIVPNSVEQKHIQKDDVPSERYLGYLEQLLRLKKYKVPQLEIVPNSAEERLHSMKEGIHAQQ 146 P47710 75 CVVAEPEKMESSISSSSEEMSLSKCA------------------EQFCRLNEYN--QLQLQAAHAQEQIRRMNENSHVQV 134 P02662 147 KEPMIGVNQELAYFYPELFRQFYQLDAYPSGAWYYVPLGTQYTDAPSFSDIPNPIGSENSEKTTMPL-W 214 ACG63494 147 KEPMIGVNQELAYFYPELFRQFYQLDAYPSGAWYYVPLGTQYTDAPSFSDIPNPIGSENSGKTTMPL-W 214 P47710 135 P-----------------FQQLNQLAAYPYAVWYY-PQIMQYVPFPPFSDISNPTAHENYEKNNVMLqW 185 Alpha-s2 casein (CSN1S2)Genetic variants & health effectsA recent review of milk protein nomenclature (Farrell et al., 2004) indicated [...] 4 αs2-CN (A, B, C, D) [...] variants in the Bos genus. [http://www.ncbi.nlm.nih.gov/pubmed/19841193] Proteolytic fragments of α-s2 Casein have been shown to exhibit antibacterial activity. Specifically the 39 amino acid casocidin-1 peptide fragment has been shown to inhibit E. coli and Staph. carnosis growth. [Zucht, H. D., FEBS Lett., 371, 185-188 (1995)] [http://www.sigmaaldrich.com/life-science/metabolomics/enzyme-explorer/enzyme-reagents/casein.html] Sequences
Bovine Alpha-s2 casein is a 222 AA protein, including a 15 AA secretion signal peptide. >sp|P02663|CASA2_BOVIN Alpha-S2-casein OS=Bos taurus GN=CSN1S2 PE=1 SV=2 MKFFIFTCLLAVALAKNTMEHVSSSEESIISQETYKQEKNMAINPSKENLCSTFCKEVVR NANEEEYSIGSSSEESAEVATEEVKITVDDKHYQKALNEINQFYQKFPQYLQYLYQGPIV LNPWDQVKRNAVPITPTLNREQLSTSEENSKKTVDMESTEVFTKKTKLTEEEKNRLNFLK KISQRYQKFALPQYLKTVYQHQKAMKPWIQPKTKVIPYVRYL In human, the alpha-s2 casein gene is duplicated, but both copies ([http://www.ncbi.nlm.nih.gov/gene/286828 CSN1S2AP], [http://www.ncbi.nlm.nih.gov/gene/100337616 CSN1S2BP ]) are truncated shortly after the signal peptide. Therefore, these loci appear to be a pseudogenes. Beta casein (CSN2)Main component of cow's and human milk. β-Casein and its fragments have been implicated in a number of biological functions. The casoparan peptide has been reported to activate macrophage phagocytosis and peroxide release. Casohypotensin and casoparan may be involved in bradykinin regulation. Casohypotensin has also been shown to be a strong inhibitor of endo-oligopeptidase A, a thiol-activated protease capable of degrading bradykinin and neurotensin, and hydrolyzing enkephalin-containing peptides to produce enkephalins. β-Caseins are also a source of casomorphin peptides which exhibit opioid activity binding to opioid receptors. Casomorphins may be the hydrolysis product of dipeptidyl peptidase IV. [Miyamoto, Y., et al., Am. J. Physiol. Renal. Physiol., 252, 670-F677 (1987)] [http://www.sigmaaldrich.com/life-science/metabolomics/enzyme-explorer/enzyme-reagents/casein.html] Genetic variants & health effectsA recent review of milk protein nomenclature (Farrell et al., 2004) indicated [...] 12 β-CN (A1, A2, A3, B, C, D, E, F, G, H1, H2, I) [...] variants in the Bos genus. [http://www.ncbi.nlm.nih.gov/pubmed/19841193] "CSN2 B positively affected milk coagulation, whereas CSN2 A(2), in particular, had a negative effect" [http://www.ncbi.nlm.nih.gov/pubmed/23746587] "Populations, which consume milk containing high levels of β-casein A2 variant, have a lower incidence of cardiovascular disease and type 1 diabetes. Furthermore, consumption of milk with the A2 variant may be associated with less severe symptoms of autism and schizophrenia." [http://www.ncbi.nlm.nih.gov/pubmed/16403684] "Human milk, goat milk, sheep milk and other species’ milk contain beta-casein which is ‘A2 like’, because they have a proline at the equivalent position in their beta-casein chains." [http://www.betacasein.org/index.php?p=variants]. The health benefits of "A2 milk" (or rather the health risks associated with A1/B beta-casein) are thought to be due to beta-casomorphin 7 (BCM-7), one of a group of degradation peptides with a chain length of 4–11 amino acids (aa), all starting with tyrosine residue in position 60 (Kostyra et al. 2004). The A1 variant (as well as B, C, F, and G) has a histidine rather than Proline in position 67, resulting in more efficient proteolysis at that location by elastase, and a 4-fold higher BCM-7 level in hydrolysed A1 milk vs A2 milk (Kaminski et al, 2007). Human BCM's also show opioid activity (Koch et al., 1985), but may have different health effects due to some amino acid differences. Sequences
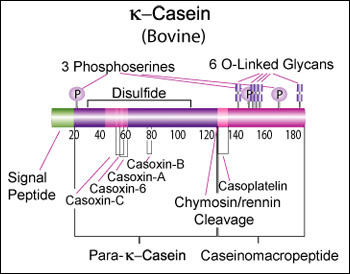
Bovine beta-casein is a 224 AA protein, including a 15 AA secretion signal. We should try bovine A2 for the reputed health benefits, and B for improved milk coagulation. A2: >gi|115660|sp|[http://www.ncbi.nlm.nih.gov/protein/P02666.2 P02666.2]|CASB_BOVIN RecName: Full=Beta-casein MKVLILACLVALALARELEELNVPGEIVESLSSSEESITRINKKIEKFQSEEQQQTEDELQDKIHPFAQT QSLVYPFPGPIPNSLPQNIPPLTQTPVVVPPFLQPEVMGVSKVKEAMAPKHKEMPFPKYPVEPFTESQSL TLTDVENLHLPLPLLQSWMHQPHQPLPPTVMFPPQSVLSLSQSKVLPVPQKAVPYPQRDMPIQAFLLYQE PVLGPVRGPFPIIV B: >gi|83406093|gb|[http://www.ncbi.nlm.nih.gov/protein/AAI11173.1 AAI11173.1]| CSN2 protein [Bos taurus] MKVLILACLVALALARELEELNVPGEIVESLSSSEESITRINKKIEKFQSEEQQQTEDELQDKIHPFAQT QSLVYPFPGPIHNSLPQNIPPLTQTPVVVPPFLQPEVMGVSKVKEAMAPKHKEMPFPKYPVEPFTERQSL TLTDVENLHLPLPLLQSWMHQPHQPLPPTVMFPPQSVLSLSQSKVLPVPQKAVPYPQRDMPIQAFLLYQE PVLGPVRGPFPIIV Human beta-casein is a 226 AA protein, including a 15 AA secretion signal peptide. >gi|115661|sp|P05814.4|CASB_HUMAN RecName: Full=Beta-casein MKVLILACLVALALARETIESLSSSEESITEYKQKVEKVKHEDQQQGEDEHQDKIYPSFQPQPLIYPFVE PIPYGFLPQNILPLAQPAVVLPVPQPEIMEVPKAKDTVYTKGRVMPVLKSPTIPFFDPQIPKLTDLENLH LPLPLLQPLMQQVPQPIPQTLALPPQPLWSVPQPKVLPIPQQVVPYPQRAVPVQALLLNQELLLNPTHQI YPVTQPLAPVHNPISV Alignment between bovine A2, bovine B, and human (55% identity over 72% of the sequence): P02666 1 MKVLILACLVALALARELEELNVPGEIVESLSSSEESITRINKKIEKFQSEEQQQTEDELQDKIHPFAQTQSLVYPFPGP 80 AAI11173 1 MKVLILACLVALALARELEELNVPGEIVESLSSSEESITRINKKIEKFQSEEQQQTEDELQDKIHPFAQTQSLVYPFPGP 80 P05814 1 MKVLILACLVALALARET---------IESLSSSEESITEYKQKVEKVKHEDQQQGEDEHQDKIYPSFQPQPLIYPFVEP 71 P02666 81 IPNS-LPQNIPPLTQTPVVVPPFLQPEVMGVSKVKEAMAPKHKEMPFPKYPVEPFTESQSLTLTDVENLHLPLPLLQSWM 159 AAI11173 81 IHNS-LPQNIPPLTQTPVVVPPFLQPEVMGVSKVKEAMAPKHKEMPFPKYPVEPFTERQSLTLTDVENLHLPLPLLQSWM 159 P05814 72 IPYGfLPQNILPLAQ-PAVVLPVPQPEIMEVPKAKDTVYTKGRVMPVLKSPTIPFFDPQIPKLTDLENLHLPLPLLQPLM 150 P02666 160 HQPHQPLPPTVMFPPQSVLSLSQSKVLPVPQKAVPYPQRDMPIQAFLLYQEPVLGP------VRGPF-----PIIV 224 AAI11173 160 HQPHQPLPPTVMFPPQSVLSLSQSKVLPVPQKAVPYPQRDMPIQAFLLYQEPVLGP------VRGPF-----PIIV 224 P05814 151 QQVPQPIPQTLALPPQPLWSVPQPKVLPIPQQVVPYPQRAVPVQALLLNQELLLNPthqiypVTQPLapvhnPISV 226 Kappa casein (CSN3)κ-Casein's orientation on the surface of the casein micelle functions as an interface between the hydrophobic interior caseins and the aqueous environment. During clotting of milk, hydrolysis by chymosin or rennin releases the water soluble fragment, para-k-casein and the hydrophobic caseinomacropeptide. [http://www.sigmaaldrich.com/life-science/metabolomics/enzyme-explorer/enzyme-reagents/casein.html]
Genetic variants & health effectsA recent review of milk protein nomenclature (Farrell et al., 2004) indicated [...] 11 κ-CN (A, B, C, E, F1, F2, G1, G2, H, I, J) [...] variants in the Bos genus. [http://www.ncbi.nlm.nih.gov/pubmed/19841193]
Sequence
Bovine kappa-casein is 190 AA of which the first 21 AA form a secretion signal. The bovine reference sequence is Genbank [http://www.ncbi.nlm.nih.gov/nuccore/AY380228 AY380228], RefSeq [http://www.ncbi.nlm.nih.gov/protein/NP_776719 NP_776719], corresponding to the CSN*A allele. Because of improved coagulation time, and possible additional health benefits, we should focus on bovine genetic variant CSN3*B instead: >gi|36988716|gb|[http://www.ncbi.nlm.nih.gov/protein/AAQ87923.1 AAQ87923.1]| kappa casein [Bos taurus] MMKSFFLVVTILALTLPFLGAQEQNQEQPIRCEKDERFFSDKIAKYIPIQYVLSRYPSYGLNYYQQKPVA LINNQFLPYPYYAKPAAVRSPAQILQWQVLSNTVPAKSCQAQPTTMARHPHPHLSFMAIPPKKNQDKTEI PTINTIASGEPTSTPTIEAVESTVATLEASPEVIESPPEINTVQVTSTAV (secretion signal in bold; caseinomacropeptide in italic) The main human variant is: >gi|1705606|sp|P07498.3|CASK_HUMAN RecName: Full=Kappa-casein MKSFLLVVNALALTLPFLAVEVQNQKQPACHENDERPFYQKTAPYVPMYYVPNSYPYYGTNLYQRRPAIA INNPYVPRTYYANPAVVRPHAQIPQRQYLPNSHPPTVVRRPNLHPSFIAIPPKKIQDKIIIPTINTIATV EPTPAPATEPTVDSVVTPEAFSESIITSTPETTTVAVTPPTA Alignment between bovine A, bovine B, and human (53% sequence identity): P02668 1 MMKSFFLVVTILALTLPFLGAQEQNQEQPIRCEKDERFFSDKIAKYIPIQYVLSRYPSYGLNYYQQKPVALINNQFLPYP 80 AAQ87923 1 MMKSFFLVVTILALTLPFLGAQEQNQEQPIRCEKDERFFSDKIAKYIPIQYVLSRYPSYGLNYYQQKPVALINNQFLPYP 80 P07498 1 -MKSFLLVVNALALTLPFLAVEVQNQKQPACHENDERPFYQKTAPYVPMYYVPNSYPYYGTNLYQRRPAIAINNPYVPRT 79 P02668 81 YYAKPAAVRSPAQILQWQVLSNTVPAKSCQAQPTTMARHPHPHLSFMAIPPKKNQDKTEIPTINTIASGEPTSTPTTEAV 160 AAQ87923 81 YYAKPAAVRSPAQILQWQVLSNTVPAKSCQAQPTTMARHPHPHLSFMAIPPKKNQDKTEIPTINTIASGEPTSTPTIEAV 160 P07498 80 YYANPAVVRPHAQIPQRQYLPN--------SHPPTVVRRPNLHPSFIAIPPKKIQDKIIIPTINTIATVEPTPAPATEPT 151 P02668 161 ESTVATLEDSPEVIE-SPPEINTVQVTSTAV 190 AAQ87923 161 ESTVATLEASPEVIE-SPPEINTVQVTSTAV 190 P07498 152 VDSVVTPEAFSESIItSTPETTTVAVTPPTA 182 CaseinomacropeptideKim YJ, Oh YK, Kang W, Lee EY, Park S. Production of human caseinomacropeptide in recombinant Saccharomyces cerevisiae and Pichia pastoris. J Ind Microbiol Biotechnol. 2005 Sep;32(9):402-8. “Caseinomacropeptide is a polypeptide of 64 amino acid residues (106-169) derived from the C-terminal part of the mammalian milk k-casein. This macropeptide has various biological activities and is used as a functional food ingredient as well as a pharmaceutical compound. The gene encoding the human caseinomacropeptide (hCMP) was synthesized and expressed with an alpha-factor secretion signal in the two yeast strains, Saccharomyces cerevisiae and Pichia pastoris. The complete polypeptide of the recombinant hCMP was produced and secreted in a culture medium by both the strains, but the highest production was observed in S. cerevisiae with a galactose-inducible promoter. In a fed-batch bioreactor culture, 2.5 g/l of the recombinant hCMP was obtained from the S. cerevisiae at 97 h.” “Escherichia coli XL1-Blue (Stratagene, USA) was used for cloning and propagating genes. Host strains for recombinant hCMP were S. cerevisiae 2805 (his-, ura-) [...] For S. cerevisiae, pYIGP (containing a constitutive GAP promoter) or pYEGa (containing a galactose-inducible GAL promoter) was used.” Role of phosphorylation and glycosylation in micelle formation or coagulation2-Dimensional electrophoresis (2-DE) can be used to distinguish κ-CN isoforms. In a study of milk from Holstein-Friesian and Jersey cows, about 1/3 of kappa-casein was glycosylated, and almost all phosphorylated. The dominant isoform was 1P (40-50%), with 2P, 1P+1G, 1P+2G and 1P+3G each around 10-15%. [http://www.sciencedirect.com/science/article/pii/S0022030212002652] We should investigate if any of this is important for micelle formation or micellar flocculation.
Casein KinaseThere are some confusing factors when talking about casein kinase. There are enzymes in most eukaryotes called Casein Kinase 1 and 2. These apparently don't have much to do with casein! The name casein kinase 2 arose from the fact that casein is a convenient protein substrate that is generally used to assay its activity ... many researchers that work with this enzyme agreed to abandon the reference to casein because it leads to confusion about a possible physiological role for this substrate. The name protein kinase CK2 was recommended. -- [http://www.ncbi.nlm.nih.gov/pubmed/7896000 Allende 1995] In fact, until 2012 the kinases responsible for casein phosphorylation were unknown. The actual kinase is a secreted kinase called Fam20C ([http://www.ncbi.nlm.nih.gov/pubmed/22582013 Tagliabracci et al. 2012], [http://www.ncbi.nlm.nih.gov/pubmed/23276407 Tagliabracci et al. 2013]). Fam20C appears to be the Golgi casein kinase that phosphorylates secretory pathway proteins within S-x-E motifs. Fam20C phosphorylates the caseins and several secreted proteins implicated in biomineralization [Casein kinase-1 and casein kinase-2] are physiologically unrelated to casein because they are mainly cytosolic and nuclear proteins that would be unlikely to encounter casein in the secretory pathway and have therefore been renamed protein kinase CK1 and protein kinase CK2. A physiological casein kinase activity has been characterized from highly enriched Golgi fractions of lactating mammary gland, liver, brain, and kidney and named Golgi-enriched fraction casein kinase (GEF-CK). The GEF-CK specifically recognizes the consensus S-x-E/pS (where x is any amino acid and E/pS can be Glu or phosphoserine). [...] A peptide substrate reproducing one of the sites phosphorylated in β-casein, KKIEKFQSE-EQQQ (β28-40), is selectively phosphorylated by GEF-CK. [...] Protein immunoblotting of immunoprecipitates from conditioned medium of U2OS cells overexpressing V5-tagged αs1-casein with FLAG-tagged Fam20C revealed a mobility shift in αs1-casein that was absent when αs1-casein was coexpressed with the inactive Fam20C D478A mutant [...] Thus, Fam20C appears to phosphorylate casein in vitro and in mammalian cells. -- [http://www.ncbi.nlm.nih.gov/pubmed/22582013/ Tagliabracci et al. 2012] Sequence
The Human FAM20C protein (also known as DMP4) is 584 AA, including a 22 AA signal peptide: >gi|327478506|sp|Q8IXL6.2|DMP4_HUMAN RecName: Full=Extracellular serine/threonine protein kinase FAM20C MKMMLVRRFRVLILMVFLVACALHIALDLLPRLERRGARPSGEPGCSCAQPAAEVAAPGWAQVRGRPGEP PAASSAAGDAGWPNKHTLRILQDFSSDPSSNLSSHSLEKLPPAAEPAERALRGRDPGALRPHDPAHRPLL RDPGPRRSESPPGPGGDASLLARLFEHPLYRVAVPPLTEEDVLFNVNSDTRLSPKAAENPDWPHAGAEGA EFLSPGEAAVDSYPNWLKFHIGINRYELYSRHNPAIEALLHDLSSQRITSVAMKSGGTQLKLIMTFQNYG QALFKPMKQTREQETPPDFFYFSDYERHNAEIAAFHLDRILDFRRVPPVAGRMVNMTKEIRDVTRDKKLW RTFFISPANNICFYGECSYYCSTEHALCGKPDQIEGSLAAFLPDLSLAKRKTWRNPWRRSYHKRKKAEWE VDPDYCEEVKQTPPYDSSHRILDVMDMTIFDFLMGNMDRHHYETFEKFGNETFIIHLDNGRGFGKYSHDE LSILVPLQQCCRIRKSTYLRLQLLAKEEYKLSLLMAESLRGDQVAPVLYQPHLEALDRRLRVVLKAVRDC VERNGLHSVVDDDLDTEHRAASAR
| |||||||||||||
Parts Table | |||||||||||||
<groupparts>iGEM014 SF_Bay_Area_DIYbio</groupparts>
 "
"