Team:Bielefeld-CeBiTec/Project/CO2-fixation/Carboxysome
From 2014.igem.org
CO2 Fixation
Carboxysome
A carboxysome is a bacterial microcompartiment (BMC) surrounded by a protein shell. Carboxysomes (CB) play an essential role in carbon fixation mechanism, as they are the enzym-containing organelles for these metabolic pathways (Bonacci et al., 2011) (Shively et al., 1973). The first carboxysomes were discovered in 1956, and purified carboxysomes were first characterized in 1973 by Shively et al., 1973.
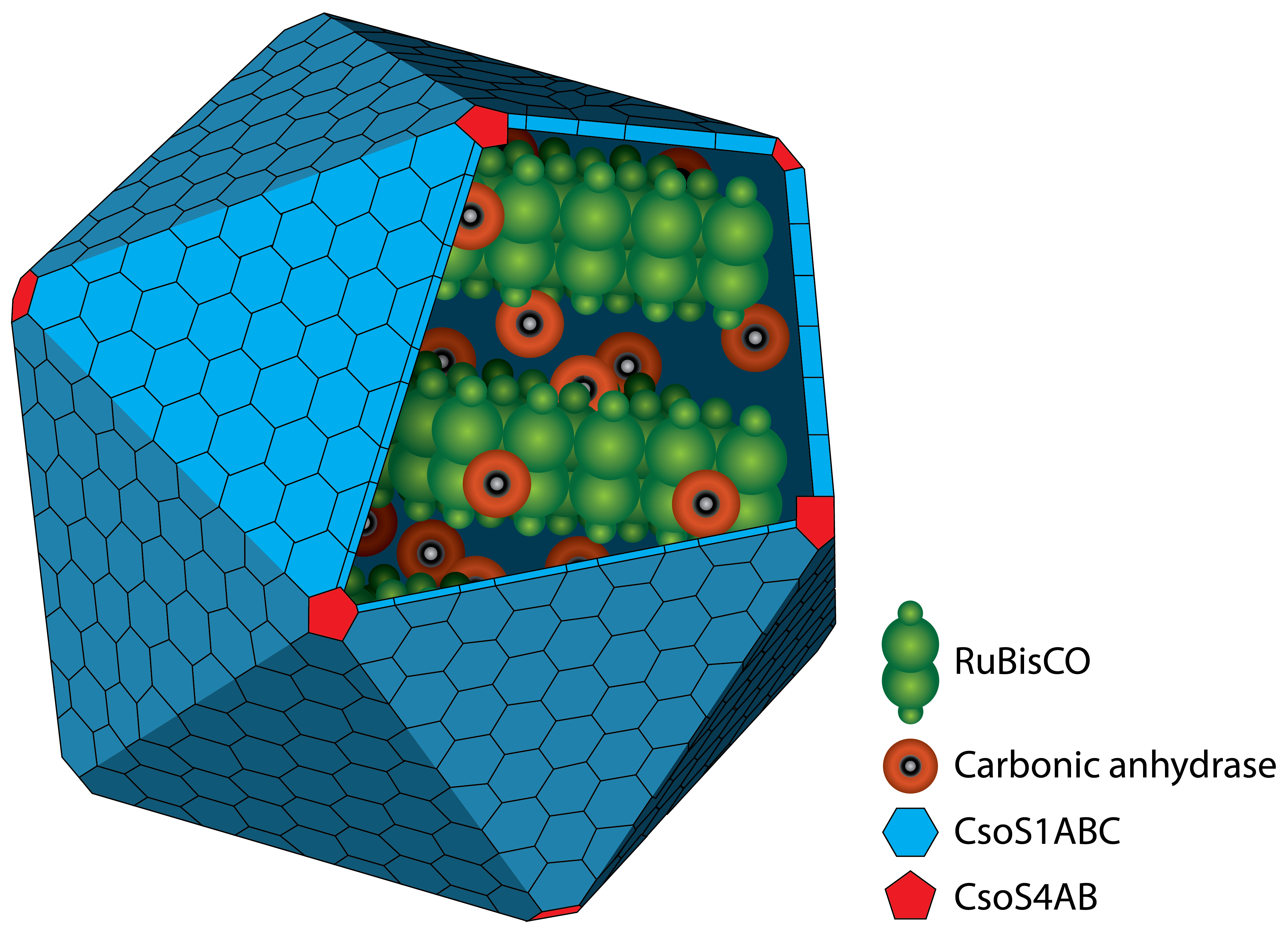
Carboxysomes, as BMCs, are formed by different shell proteins, surrounding an enzyme-containing lumen, which is rigorous seperated from the cytoplasm. Thus, the possibility is given to enable reaction pathways incongruous with the conditions in the cytoplasm.
The protein shell consists of two different types of proteins. Pentamers are used for the vertices of the icosaeder and hexamers for the facets. The main constituent of the shell is the CsoS1A protein, 9.9 kDa large, which makes arround 13 % of the total protein mass). Carboxysomes are between 80 and 120 nm in diamter (Kehrfeld et al., 2005)). In the interior, there are two different types of enzymes. On the one hand there is the
RuBisCo which catalyses the carboxylation or oxygenation of ribulose-1,5-bisphosphate. On the other hand there is the carbonic anhydrase which converts hydrogen carbonate (HCO3-) to carbon dioxide. The resulting carbon dioxide is the substrate for the RuBisCO.
Generally, there are two different types of CBs found, called α-type and β-type carboxysomes. This classification is based on the differences in their component proteins and organisation of their corresponding genes.
α-type carboxysomes are found in α-cyanobacteria like some Synechococcus species or the chemoautotroph model organism Halothiobacillus neapolitanus. This type of carboxysome contains the type I RuBisCo. Furthermore the respective genes for the carboxysome are arranged in a single operon. In β-type carboxysomes occurs the type II RuBisCo, containing no small subunits. In contrast to α-type carboxysomes, the genes are localisated in multiple gene clusters. (Bonacci et al., 2011) (Yeates et al., 2008) A possible difference in functionality between both types of carboxysomes is not fully understood (Yeates et al., 2008).
The advantage of the microcompartiment is the concentration of carbon dioxide in its lumen. Carboxyomes play a central role in carbon concentrating mechanism (CCM). This mechanism is used for accumulation of CO2 in autotrophic prokaryotes. The advantage is that the concentration of carbon dioxide inside the carboxysome can be much higher than outside which increases the efficiency of the RuBisCO-catalyzed reaction. The first reaction of the CCM is the transport of atmospheric carbon dioxide or Bicarbonate (HCO3-) into the cells. Accumulation of inorganic carbon is archieved by transmembrane pumps and transporters in the cell membrane. They exist in low and high affinity forms. At physiological ph values (approximate 7), the balance is clearly on the side of bicarbonate. Bicarbonate is able to diffuse across the shell of the carboxysome. The carbonix anhydrase (CA), located inside the carboxysome, catalyses the conversion of bicarbonate to the substrate for RuBisCo, gaseous carbon dioxide. The CA is assosciated with the shell and presents only a small number of the carboxysome proteins. Thus, the CA, also named as CSOS3, acts to saturate the lumen with carbon dioxide and enables high effective concentrations of CO2, so that the RuBisCo is able to work near its maximal reaction rate with high specifity.
References
-
Batger,Price 2011. CO2 concentrating mechanisms in cyanobacteria: molecular components, their diversity and evolution Journal of Experimental Botany, vol. 54, pp. 609-622
-
Batger,Price 2003. CO2 concentrating mechanisms in cyanobacteria: molecular components, their diversity and evolution Journal of Experimental Botany, vol. 54, pp. 609-622
-
Bonacci et al., 2011. Modularity of carbon-fixing protein organelle. PNAS, vol. 109, pp. 478-483
-
Kerfeld et al., 2005. Protein structures Forming the Shell of Primitative Bacterial Organelles. Science, vol. 309, pp. 936-938
-
Yeates et al., 2011. Protein-based organelles in bacteria: carboxysomes and related microcompartments. Nature Reviews Microbiology, vol. 6, pp. 681-691
 "
"