Team:ETH Zurich/lab/bead
From 2014.igem.org
(→Properties) |
(→Overview) |
||
| Line 5: | Line 5: | ||
{| | {| | ||
| - | [[File:ETH2014_BeadLogo.jpg|left|300px|thumb| '''Figure 1 Na<sup>2+</sup> alginate is extruded through a needle device | + | [[File:ETH2014_BeadLogo.jpg|left|300px|thumb|'''Figure 1''' Na<sup>2+</sup> alginate is extruded through a needle device]] |
Alginates are present as structural components in both the cell walls of brown algae and the capsules of soil bacteria. However, commercially available alginate is mainly extracted from algae. The polysaccharides find a broad application in various fields: in textile printing, in food industry or in research. The chemical features of alginates allow immobilization of macromolecules and cells, thus, the compound is commonly used in biotechnology, biomedicine and pharmacy<sup>[[Team:ETH_Zurich/project/references#refEmergence|[24]]]</sup>. In our project we encapsulated bacteria in alginate beads so as to ensure local separation of the different units (here strains) and directional communication between them. These prerequisites are required for controlled pattern formation. | Alginates are present as structural components in both the cell walls of brown algae and the capsules of soil bacteria. However, commercially available alginate is mainly extracted from algae. The polysaccharides find a broad application in various fields: in textile printing, in food industry or in research. The chemical features of alginates allow immobilization of macromolecules and cells, thus, the compound is commonly used in biotechnology, biomedicine and pharmacy<sup>[[Team:ETH_Zurich/project/references#refEmergence|[24]]]</sup>. In our project we encapsulated bacteria in alginate beads so as to ensure local separation of the different units (here strains) and directional communication between them. These prerequisites are required for controlled pattern formation. | ||
|} | |} | ||
| Line 17: | Line 17: | ||
{| | {| | ||
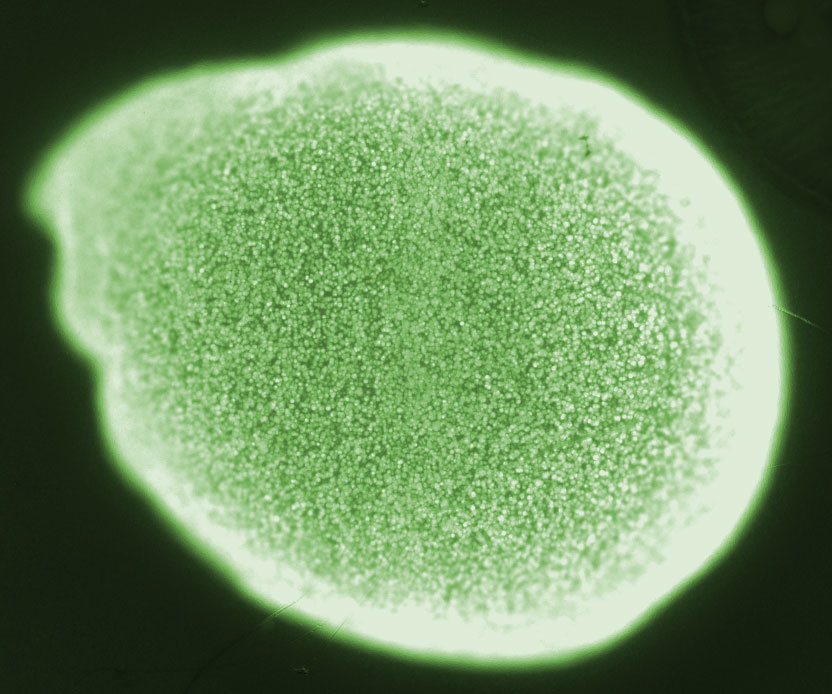
| - | [[File:ETH2014_BeadColonies.jpg|right|300px|thumb| '''Figure 2 The green spots are ''E. coli'' colonies inside an alginate bead | + | [[File:ETH2014_BeadColonies.jpg|right|300px|thumb| '''Figure 2''' The green spots are ''E. coli'' colonies inside an alginate bead]] |
Na<sup>2+</sup> alginate is a viscous liquid, however, upon addition multivalent ions such as Ca<sup>2+</sup> cross-linking of the polysaccharides occurs. Thus gelling of alginate can be achieved by the addition of a Ca<sup>2+</sup>. For encapsulation the cells or macromolecules are added to Na<sup>2+</sup> alginate and subsequently immobilized during the gelling process. In fact, the encapsulation process is mild and compatible with most living cells. The high porosity of the ionically cross-linked polysaccharide lattice allows diffusion of nutrients and other substances into and out of the bead. This property of Ca<sup>2+</sup> alginate allows cultivation of bacteria inside beads and does not prevent communication via small molecules between colonies of different beads. Substances such as phosphate or EDTA are sequestrating Ca<sup>2+</sup> and thus destabilizing the alginate gel<sup>[[Team:ETH_Zurich/project/references#refEmergence|[25]]]</sup>. This fact should be considered when choosing the cultivation medium. Beads are permeable for our signal molecules HSL, which allows bead to bead communication. | Na<sup>2+</sup> alginate is a viscous liquid, however, upon addition multivalent ions such as Ca<sup>2+</sup> cross-linking of the polysaccharides occurs. Thus gelling of alginate can be achieved by the addition of a Ca<sup>2+</sup>. For encapsulation the cells or macromolecules are added to Na<sup>2+</sup> alginate and subsequently immobilized during the gelling process. In fact, the encapsulation process is mild and compatible with most living cells. The high porosity of the ionically cross-linked polysaccharide lattice allows diffusion of nutrients and other substances into and out of the bead. This property of Ca<sup>2+</sup> alginate allows cultivation of bacteria inside beads and does not prevent communication via small molecules between colonies of different beads. Substances such as phosphate or EDTA are sequestrating Ca<sup>2+</sup> and thus destabilizing the alginate gel<sup>[[Team:ETH_Zurich/project/references#refEmergence|[25]]]</sup>. This fact should be considered when choosing the cultivation medium. Beads are permeable for our signal molecules HSL, which allows bead to bead communication. | ||
|} | |} | ||
| Line 28: | Line 28: | ||
===Production=== | ===Production=== | ||
{| | {| | ||
| - | [[File:ETH2014_BeadsProduction.jpg|left|250px|thumb| '''Figure 3 | + | [[File:ETH2014_BeadsProduction.jpg|left|250px|thumb| '''Figure 3''' Bead production. Alginate droplets are gelling in the CaCl<sub>2</sub> solution in the beaker placed under the needle device]] |
Bacteria are resuspended and diluted in NaCl-solution (0.9 % in H<sub>2</sub>O) so as to achieve the desired cell density. Here, we aimed at a comparably high concentration of 10<sup>7</sup> bacteria per bead (3 mm diameter). The resuspension was added to alginate (2.5%) to reach a alginate concentration of 2%. The viscous solution is filled into a syringe containing an appropriate needle device and extruded at a constant, slow velocity. Ideally, the droplets should fall form a height of approximately 30 cm into a 100 mM CaCl<sub>2</sub> solution. In the CaCl<sub>2</sub> solution gelling of the alginate droplets will occur instantaneously. To avoid excessive salt stress for the bacteria the beads should be transferred to a 10 mM CaCl<sub>2</sub> solution. | Bacteria are resuspended and diluted in NaCl-solution (0.9 % in H<sub>2</sub>O) so as to achieve the desired cell density. Here, we aimed at a comparably high concentration of 10<sup>7</sup> bacteria per bead (3 mm diameter). The resuspension was added to alginate (2.5%) to reach a alginate concentration of 2%. The viscous solution is filled into a syringe containing an appropriate needle device and extruded at a constant, slow velocity. Ideally, the droplets should fall form a height of approximately 30 cm into a 100 mM CaCl<sub>2</sub> solution. In the CaCl<sub>2</sub> solution gelling of the alginate droplets will occur instantaneously. To avoid excessive salt stress for the bacteria the beads should be transferred to a 10 mM CaCl<sub>2</sub> solution. | ||
<br> | <br> | ||
| Line 40: | Line 40: | ||
===Loading the Chip=== | ===Loading the Chip=== | ||
{| | {| | ||
| - | [[File:ETH_Zurich_2014_Beadloding_with_mobile_phone.jpg|right|350px|thumb| '''Figure 4 Loading of the chip | + | [[File:ETH_Zurich_2014_Beadloding_with_mobile_phone.jpg|right|350px|thumb| '''Figure 4''' Loading of the chip. The cell phone light facilitates to locate the beads loaded in the PDMS chip]] |
The beads are better visible in liquid when light shines from the side. So it is easier to load the [https://2014.igem.org/Team:ETH_Zurich/lab/chip the chip] with help of a cell phone light. An inoculation loop with a diameter of around 2 mm can be used to conveniently transfer the beads. | The beads are better visible in liquid when light shines from the side. So it is easier to load the [https://2014.igem.org/Team:ETH_Zurich/lab/chip the chip] with help of a cell phone light. An inoculation loop with a diameter of around 2 mm can be used to conveniently transfer the beads. | ||
|} | |} | ||
Revision as of 23:59, 17 October 2014
Beads
Overview
Properties
Production
Loading the Chip
 "
"