Team:Aachen/Project/Model
From 2014.igem.org
(→Modeling) |
AZimmermann (Talk | contribs) (→Modeling) |
||
| Line 13: | Line 13: | ||
</center> | </center> | ||
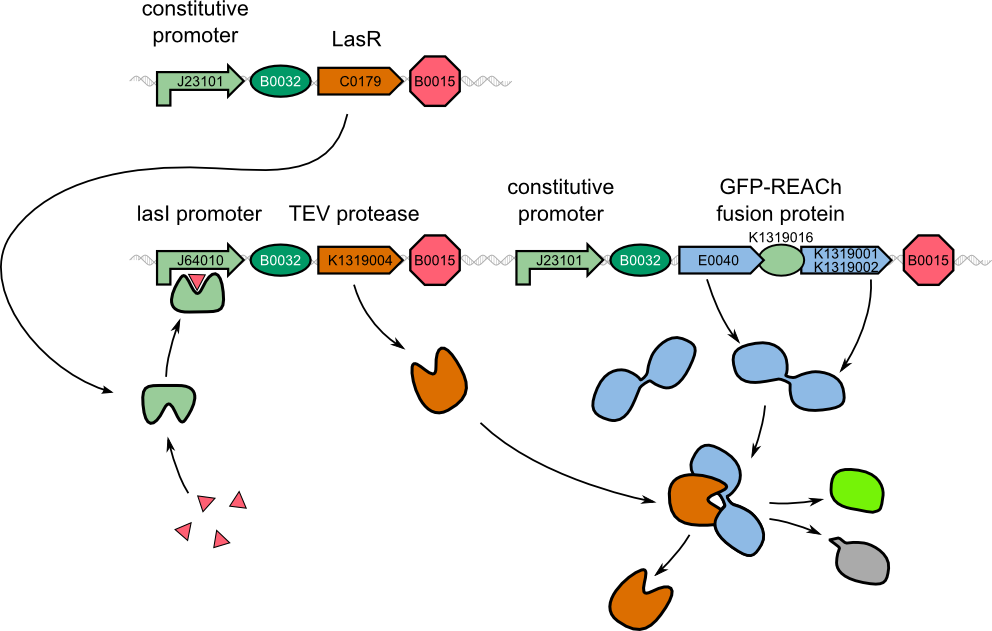
| - | For our two-dimensional biosensor, we thought of different methods to generate faster and stronger fluorescence responses from weak | + | For our two-dimensional biosensor, we thought of different methods to generate faster and stronger fluorescence responses from weak promoters. We were inspired by a recently published engineered ''dark quencher'', called REACh, that is able to extinguish the fluorescence of GFP. In our system, we wanted a fusion protein of GFP with the dark quencher to be cleaved by the very specific TEV protease that would then be introduced behind the weak quorum sensing promoter. |
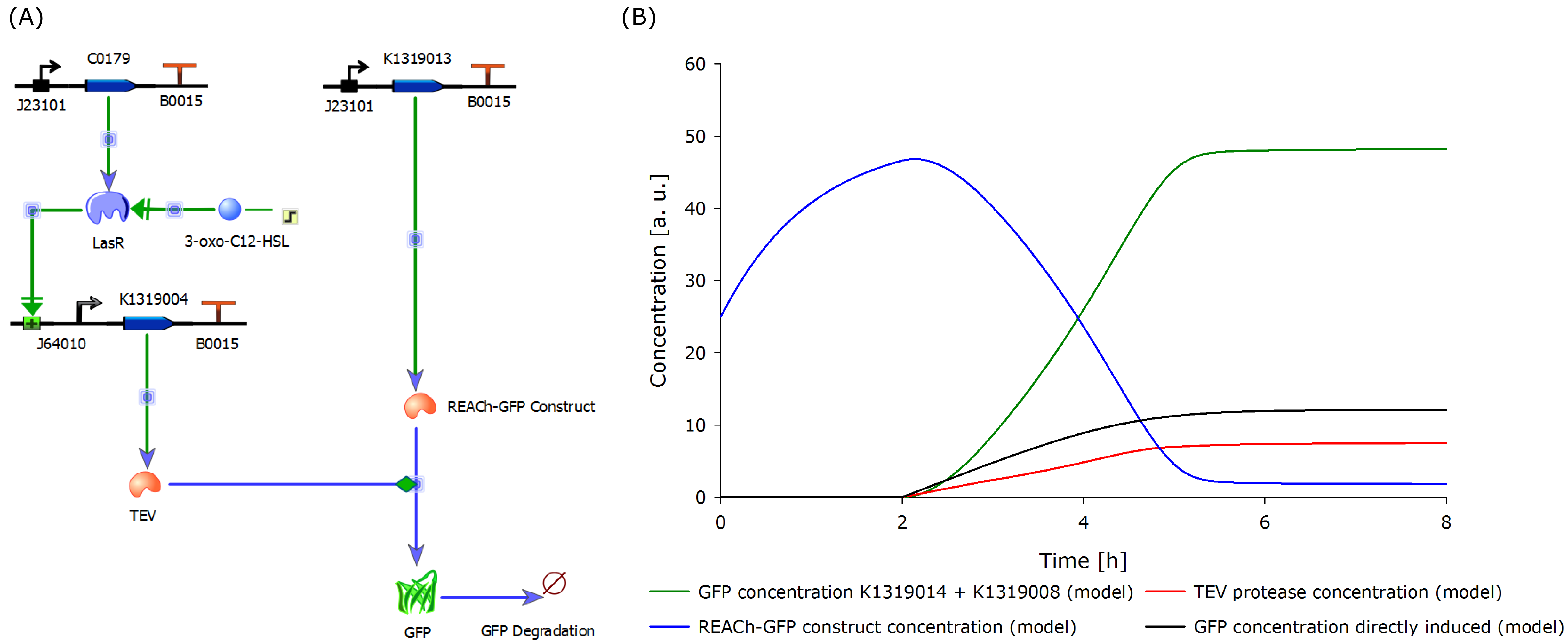
To determine if this idea was actually feasible, we decided to model the system using the CAD tool TinkerCell (Chandran, Bergmann and Sauro, 2009). | To determine if this idea was actually feasible, we decided to model the system using the CAD tool TinkerCell (Chandran, Bergmann and Sauro, 2009). | ||
| - | To compare the response time of the fluorescence signal between our theoretical system and a traditional biosensor, we included a direct expression of GFP in the same plot (below). In the results | + | To compare the response time of the fluorescence signal between our theoretical system and a traditional biosensor, we included a direct expression of GFP in the same plot (below). In the results shown below, the strength of the promotor used for the direct GFP expression (traditional approach) is even twice as high as the strength of the promoter upstream of the TEV coding sequence in our new approach. Despite the weaker promoter, a '''higher GFP concentration is generated in the model of the novel biosensor''', predicting a quicker response time of our system. |
| Line 25: | Line 25: | ||
| - | The model predicted that our approach should be an improvement over the commonly used direct expression, so we | + | The model predicted that our approach should be an improvement over the commonly used direct expression, so we proceeded with the clonings and assembled plasmids to test the system. |
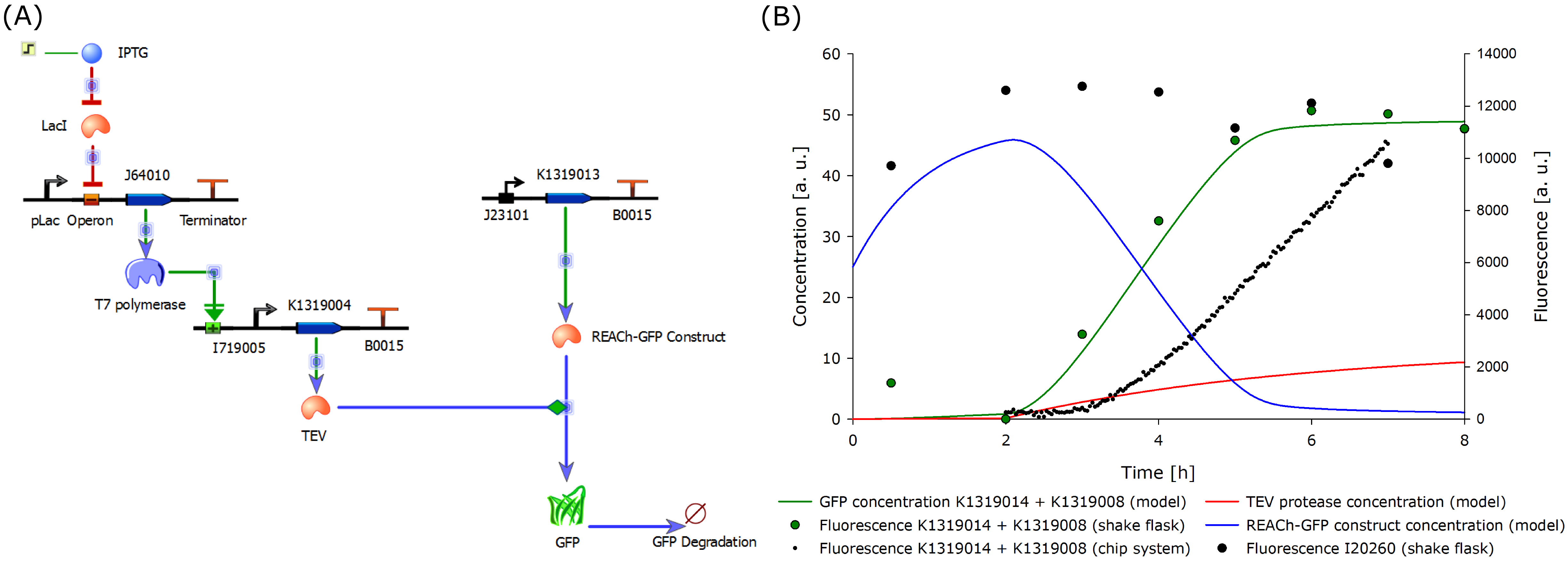
As the first experimental data came in, we correlated the experimental data gathered from the characterization of the double plasmid system K1319014 + K1319008 to the model. Due to the complexity of the quorum sensing circuit, we assembled an IPTG-inducible TEV protease instead of the 3-oxo-C<sub>12</sub>-HSL-inducible version. | As the first experimental data came in, we correlated the experimental data gathered from the characterization of the double plasmid system K1319014 + K1319008 to the model. Due to the complexity of the quorum sensing circuit, we assembled an IPTG-inducible TEV protease instead of the 3-oxo-C<sub>12</sub>-HSL-inducible version. | ||
| Line 36: | Line 36: | ||
| - | The model was fitted to the data gathered from the characterization experiment conducted in shake flasks. Additionally, the data from the characterization experiment of the double plasmid construct K1319014 + K1319008 in the chip system was included in the plot. The data was derived from the plate reader output of the four central spots of the chip. The background from the | + | The model was fitted to the data gathered from the characterization experiment conducted in shake flasks. Additionally, the data from the characterization experiment of the double plasmid construct K1319014 + K1319008 in the chip system was included in the plot. The data was derived from the plate reader output of the four central spots of the chip. The background from the non-induced chip was substracted from the fluorescent response to correct the data and avoid effects from cell growth leading to wrong signal strengths. The development of the fluorescence is presented [https://2014.igem.org/Team:Aachen/Project/FRET_Reporter#reachachievementschip here]. |
It is shown that the fluorescent response in chips occurs later than in the characterization experiment in shake flasks. This is explainable as the solid agar chip poses a greater diffusion barrier than liquid medium as used in the shake flasks. Further, the rate of fluorescence increase over time is smaller than in the characterization experiments in shake flasks. The reason is that the sensor cells need oxygen to produce GFP. However, they are embedded in solid agar in which the amount of available oxygen is lower compared to a shaking liquid system. | It is shown that the fluorescent response in chips occurs later than in the characterization experiment in shake flasks. This is explainable as the solid agar chip poses a greater diffusion barrier than liquid medium as used in the shake flasks. Further, the rate of fluorescence increase over time is smaller than in the characterization experiments in shake flasks. The reason is that the sensor cells need oxygen to produce GFP. However, they are embedded in solid agar in which the amount of available oxygen is lower compared to a shaking liquid system. | ||
Revision as of 23:14, 17 October 2014
|
|
 "
"