Achievements
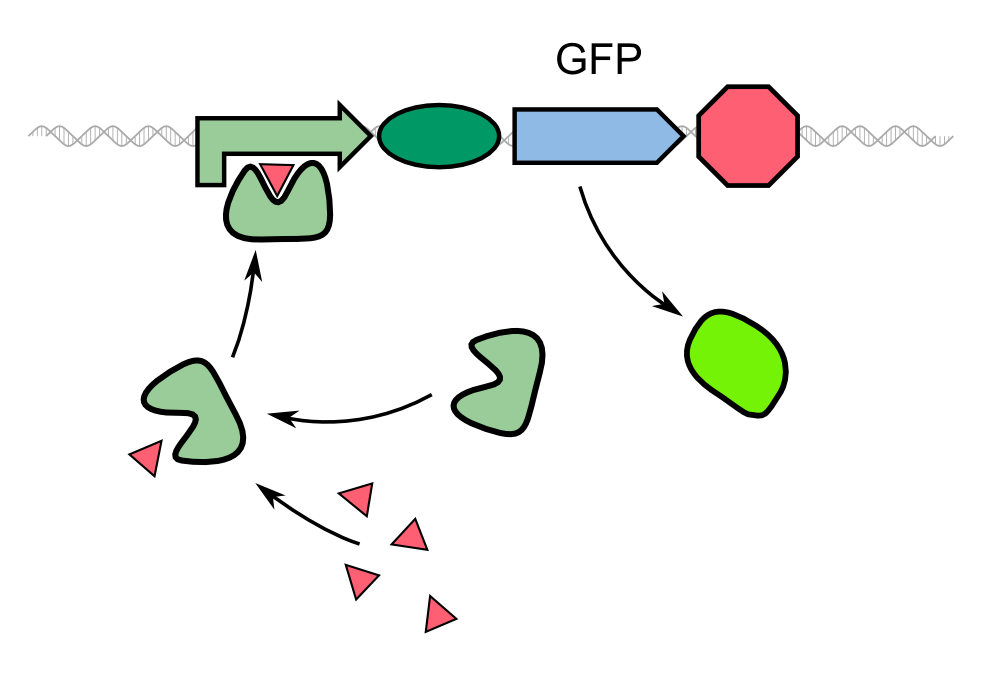
Characterization of GFP-REACh1 and GFP-REACh2 fusion proteins in combination with an IPTG-inducible TEV Protease
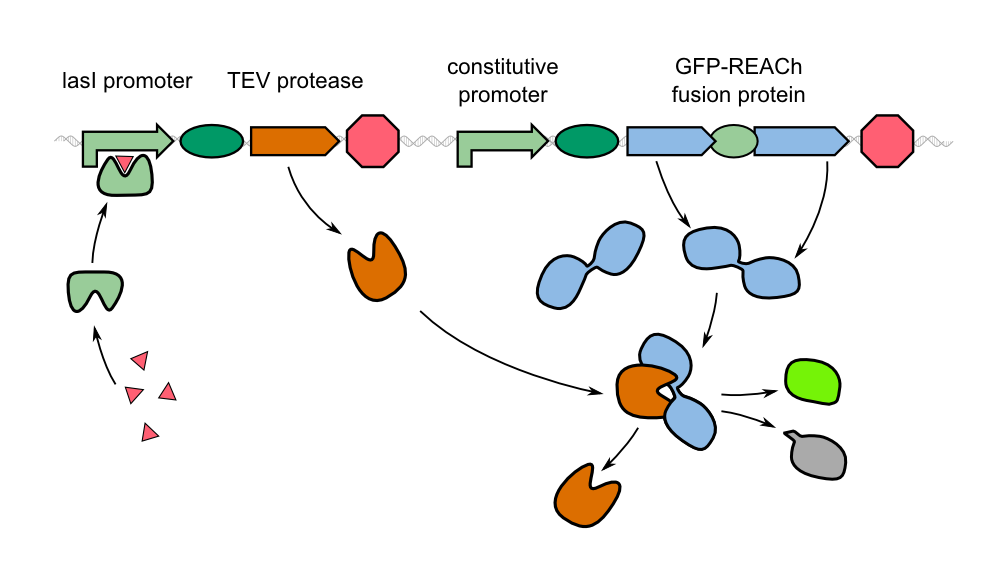
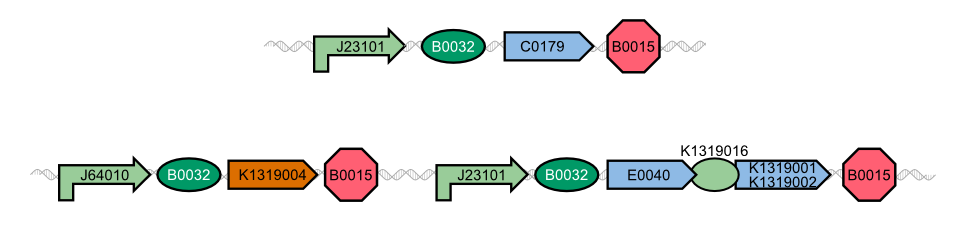
The characterization of the TEV protease and the REACh1 and REACh2 dark quenchers was performed by introducing both simultaneously into E. coli Bl21 (DE3). The resulting double plasmid cells therefore contained [http://parts.igem.org/Part:BBa_K1319013 K1319013] (GFP-REACh1 fusion protein) or [http://parts.igem.org/Part:BBa_K1319014 K1319014] (GFP-REACh2 fusion protein) and [http://parts.igem.org/Part:BBa_K1319008 K1319008] (IPTG-inducible TEV protease). K1319013 and K1319014 were located on a pSB3K3 plasmid backbone and K1319008 on a pSB1C3 backbone, two standard iGEM plasmids with different oris allowing simultaneous use in one cell.
[http://parts.igem.org/Part:BBa_I20260 I20260] was used as a positive control because I20260 contains the same promoter ([http://parts.igem.org/Part:BBa_J23101 J23101]), the same RBS ([http://parts.igem.org/Part:BBa_B0032 B0032]) and the same version of GFP ([http://parts.igem.org/Part:BBa_E0040 E0040]) and is located on the same plasmid backbone pSB3K3. Therefore, it is expected that when all fusion proteins are successfully cut by the TEV protease, the fluorescence level of the double plasmid constructs reaches the same level as the positive control of I20260. As a negative control[http://parts.igem.org/Part:BBa_B0015 B0015] was used, a coding sequence of a terminator which should not show any sign of fluorescence.
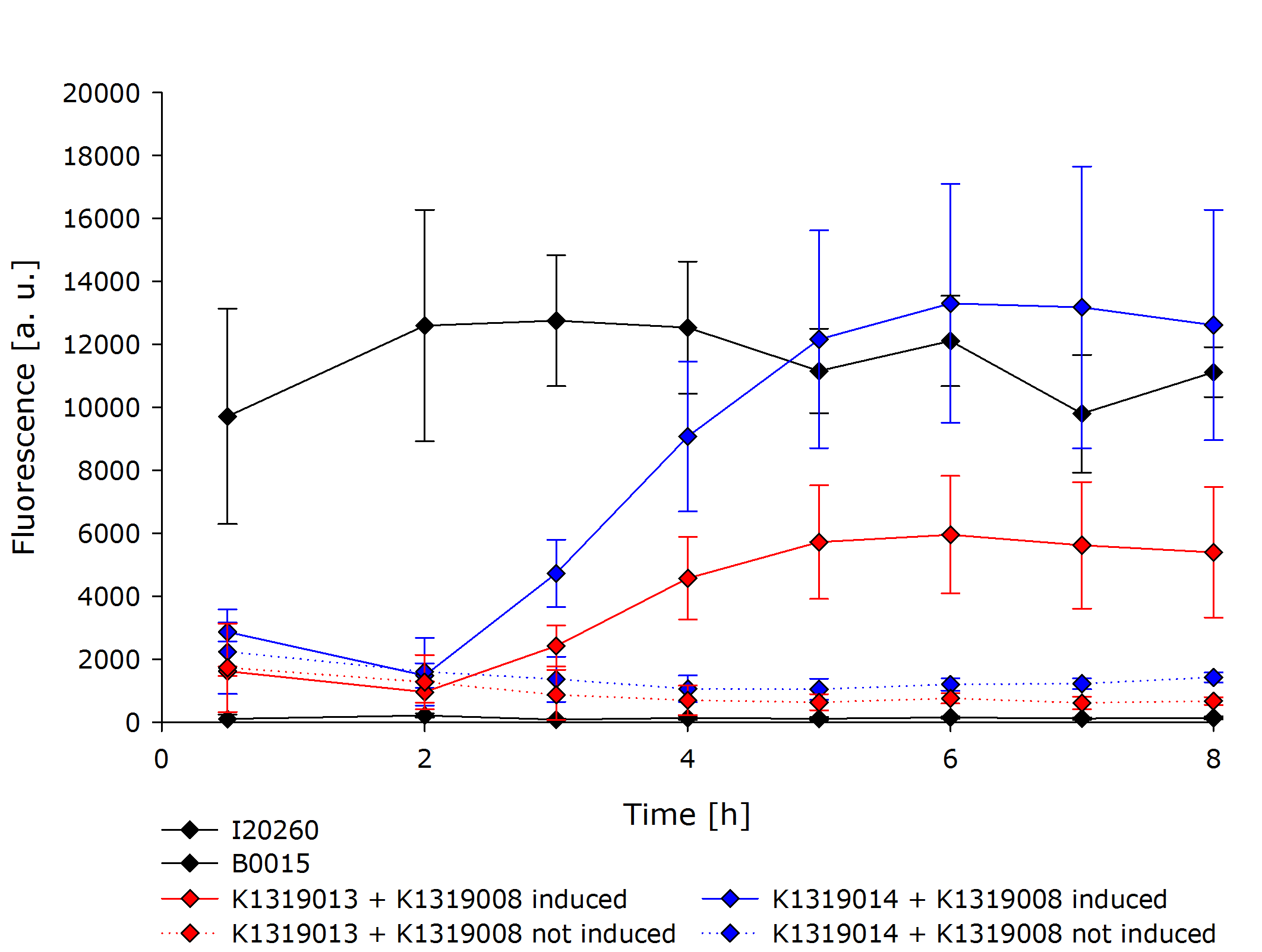
To characterize our REACh1/2 constructs in combination with the TEV protease a growth experiment was conducted. Both of the double plasmid constructs, a constitutive expression of GFP (I20260) as positive control and B0015 as negative control were compared. For each expression IPTG-induced and uninduced cultures were grown in parallel. All measurements were done in a biological triplicate.
To better evaluate the fluorescence, the observed Optical desity (OD) was taken into account in order to achieve a fluorescence measurement independent of the amount of cells present. This way, the measurement represents the amount of fluorescence per cell only.
The negative control B0015 did not exhibit any significant fluorescence. The positive control I20260 showed a steady level of fluorescence as expected due to the constitutive expression of GFP. As expected, the production is also independent of addition of IPTG therefore the triplicates have been merged together.
Both double plasmid constructs K1319013 + K1319008 and K1319014 + K1319008 did not exhibit a strong fluorescence before induction with IPTG. In the uninduced state, the fluorescence stays low and does not increase over time. It is significantly weaker than the fluorescence reached by the induced constructs or the positive control but was higher than the negative control. The higher base level of fluorescence in the not induced constructs is due to the imperfect quenching and the leakiness of the IPTG inducible promoter.
The induced double plasmid constructs exhibited a fast rise in fluorescence after induction. The signal strenght increased ~ 10-fold over the uninduced constructs. K1319014 + K1319008 reached the the same level of fluorescence as I20260, indicating a complete cleavage of the fusion proteins by the TEV protease. K1319013 + K1319008 did not reach a level of fluorescence as high as K1319013 + K1319008, however, the nearly 10-fold increase in fluorescence after induction is a clear indicator for the TEV protease cutting the fusion protein K1319013. The weaker fluorescence signal is probably due to a lower expression level of K1319013 in the cells compared to the expression level of K1319014.
The fluorescence results of K1319014 + K1319008 was used to fit our model to experimental data.
Summary
The double plasmid systems of K1319013 + K1319008 and K1319014 + K1319008 demonstrate the quenching ability of the REACh1 and REACh2 proteins as well as the funcionality of the TEV protease.
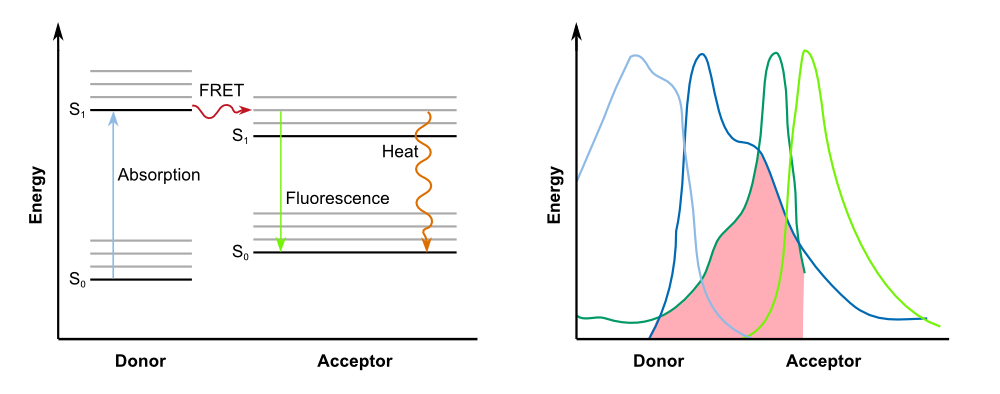
The increase in fluorescence after induction with IPTG is based on a functional expression of the TEV protease which proceeds to cut the linker of the fusion protein already produced destroying the FRET system between GFP and its quencher and resulting in a strong fluorescence signal. Combined, this characterization is a validation of the functionality of the REACh1 protein ([http://parts.igem.org/Part:BBa_K1319001 K1319001]), the REACh2 protein ([http://parts.igem.org/Part:BBa_K1319002 K1319002]) and the TEV protease ([http://parts.igem.org/Part:BBa_K1319004 K1319004]).
Determining the Quenching ability of REACh 1 and REACh 2
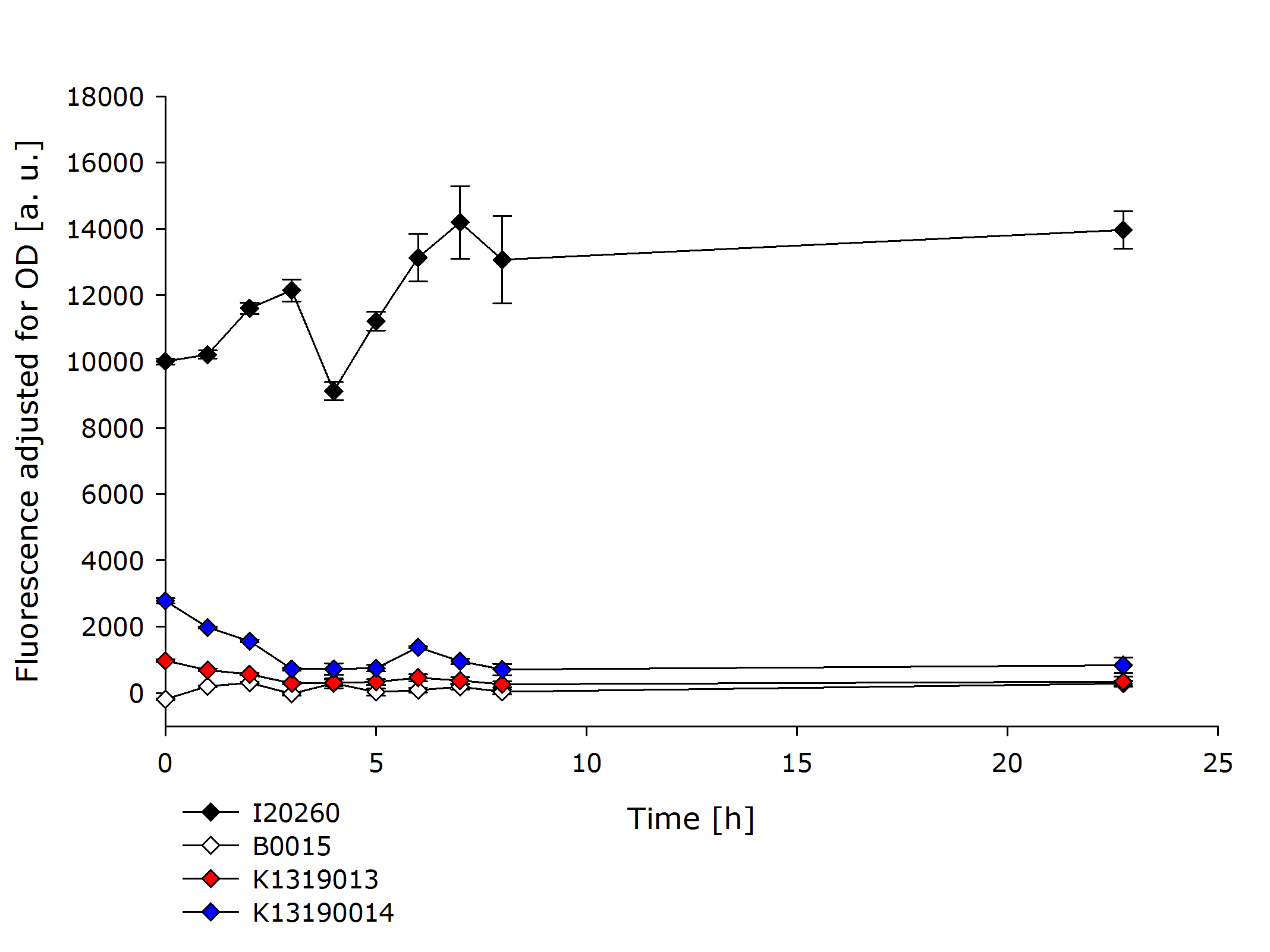
In order to further evaluate the quenching ability of the REACh 1 and REACh 2 constructs in the fusion proteins produced by K1319013 and K1319014, they were expressed alone without an IPTG inducible TEV protease. This eliminated the effect of a potential leakiness of the non induced promoter to reliably assess the quenching ability of the REACh 1 and REACh 2 proteins.
In the previous experiment it was established that the fusion proteins K1319013 and K1319014 are expressed funtionally. K1319014 reached the same level of fluorescence as the positive control after being cut by the TEV protease. Therefore the reduced fluorescence in this experiment is completely attributable to the quenching of REACh 1. The quenching reduces the fluorescence of GFP by a factor ~ 25 which means a quenching efficiency of ~ 96%!.
K139013 was not able to reach the same fluorescence level as the positive control in the previous experiment. Therefore a worse rate of functional expression of K1319013 compared to K1319014 is assumed. incorporating this, the difference in fluorescence between induced and not induced is still a factor of ~ 30 resulting in a quenching efficiency of ~ 97%.
Summary
The fusion proteins of GFP combined with REACh 1 and REACh 2 are not only fully functional but exhibit a great quenching efficiency of ~ 97% for REACh 1 and ~ 96% for REACh 2.Even though REACh 1s quenching ability seems to be slighty superior, the expression level of the K1319014 fusion protein including REACh 2 is nearly twice as high as the fusion protein K1319013 containing REACh 1 and therefore shows a stronger fluorescence. Ganesan et al. (2006) reported a reduction on emission of GFP of 82% for REACh 1 and 98% for REACh 2 but with a different Linker between the proteins and on a different vector backbone.
Comparing fluorescence kinetics of the GFP-REACh fusion proteins with a Standard lacI-inducible GFP Expression
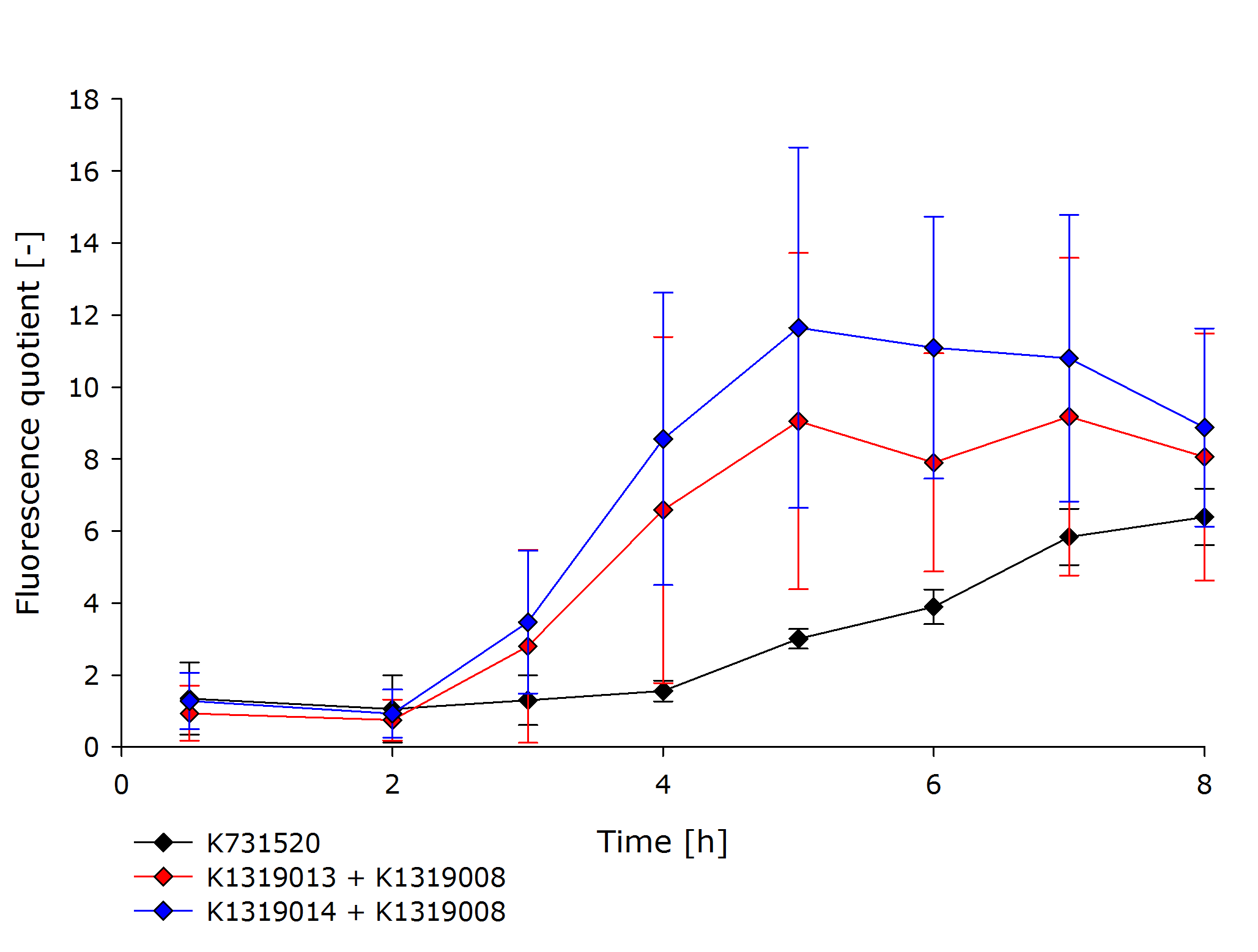
To assess the kinetics of the fusion proteins K1319013 (GFP-REACh1) and K1319014 (GFP-REACh2), the double plasmid systems K1319013 + K1319008 and K1319014 + K1319008 were compared to a standard expression of GFP under the control of a lacI promoter in [http://parts.igem.org/Part:BBa_K731520 K731520], a BioBrick made by the iGEM Team TRENTO in 2012. This tested the hypothesis of achieving a faster fluorescence response with the GFP-REACh fusion proteins compared to a standard expression.
K731520 and the double plasmid constructs K1319013 + K1319008 and K1319014 + K1319008 were cultivated in E. coli BL21(DE3), and fluorescence and OD was measured.The fluorescence was adjusted for the OD to show a relative fluorescence on a per cell basis. The difference between the induced and uninduced state, the fluorescence quotient, serves as a better indicator for a system used as a sensor because the difference between an on and off state is more important for a clear and unmistakable signal than the overall fluorescence. Hence, the OD-adjusted fluorescence quotient for both double plasmid constructs and K731520 was obtained and plotted in the following graph.
The graph clearly shows the faster response of the cut GFP-REACh fusion protein compared to a standard GFP expression. Both fluorescence signals of the double plasmid constructs achieve a higher difference in fluorescence signal between induced and uninduced state as well as at a faster rate. This proves the hypothesis made earlier about the kinetics of the GFP-REACh fusion protein combined with the TEV protease.
Summary
The kinetics of the fusion protein combined with the TEV protease exhibits the exact characteristics as predicted. The response is clearly faster than normal expression by accumulating a reservoir of fusion proteins which are not fluorescing due to the dark quencher attached to them. This reservoir is then activated by the induction of the TEV protease expression. Production of the protease results in the cleavage of the fusion protein, releasing GFP from the dark quencher and disturbing the interaction between the FRET pair. This results in the observed faster fluorescence reaction due to the amplificating effect of the TEV protease in which every one TEV protease can account for many fluorescence proteins being activated.
Characterizing the GFP-REACh Constructs in Sensor Chips
To further characterize the REACh construct, they were introduced into the sensor cells which were then induced with 2 µL IPTG with a concentration of 100 mM. Subsequently, we took fluorescence measurement read-outs (GFP, excitation 496 ± 9 nm, emission 516 ± 9 nm) roughly every 10 min in the plate reader. The results were plotted in the heatmap shown here.
The heatmap shows an increase of fluorescence from blue (no fluorescence) to red (high fluorescence). It is clearly visible that the induced chips are exhibiting a significantly higher fluorescence than the uninduced chips. This shows that the constructs also work as intended in the sensor chips: The TEV protease cuts the linker so that the fusion protein is separated into GFP and a dark quencher, disabling the quenching. GFP has a clear fluorescence emission after the fusion protein has been successfully cut into two pieces by the TEV protease.
Comparing the kinetic of the double plasmid systems K1319013 + K1319008 and K1319014 + K1319008 with standard GFP expression
The analysis of the different sensor chips with the three different construct K1319013 + K1319008, K1319014 + K1319008 and K731520 demonstrates the same fluorescence response kinetic as in the shake flask experiments. The double plasmid systems exhibits a faster and stronger fluorescence response compared to a standard GFP expression in K731520. The build up pool of fusion proteins allows for a faster, stronger fluorescence response when the induced TEV protease cleaves the fusion proteins and releases GFP from its dark quencher.
|
 "
"