Team:Aachen/Project/FRET Reporter
From 2014.igem.org
(→Cutting the Fusion Protein with the TEV Protease) |
(→Achievements) |
||
| Line 165: | Line 165: | ||
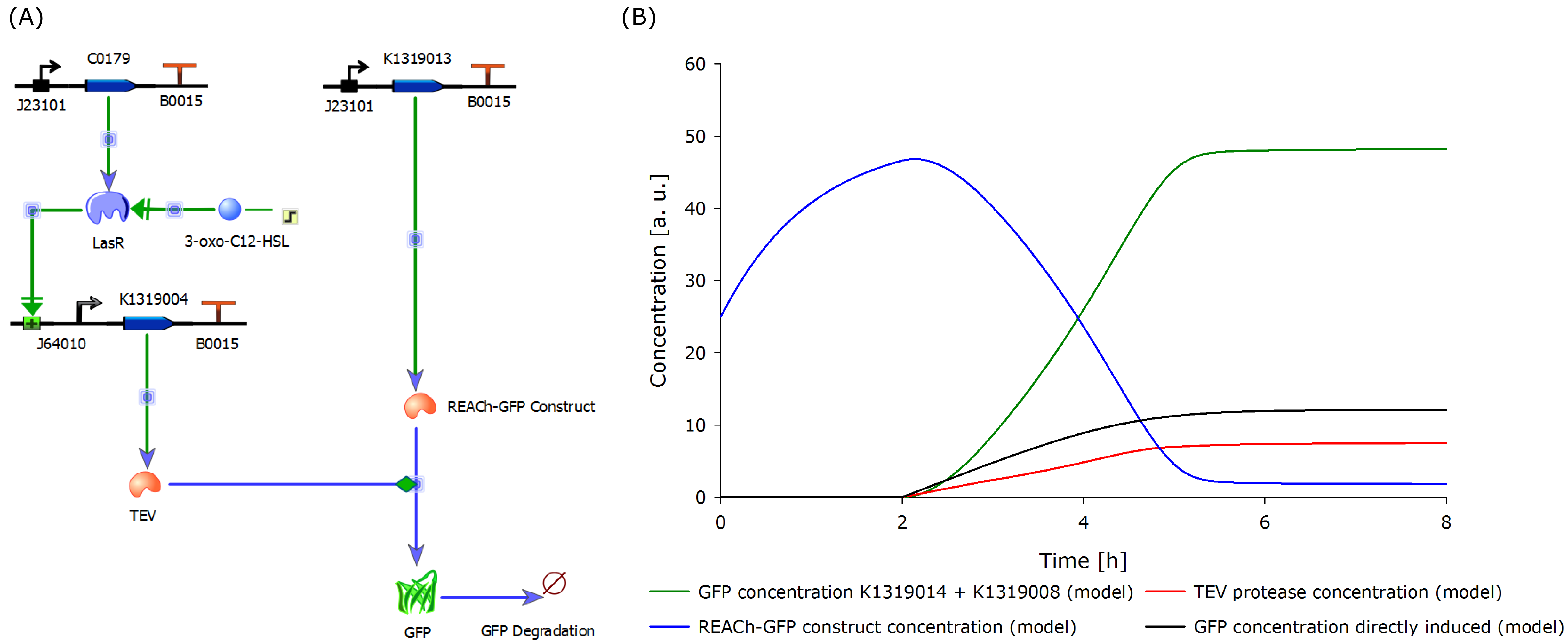
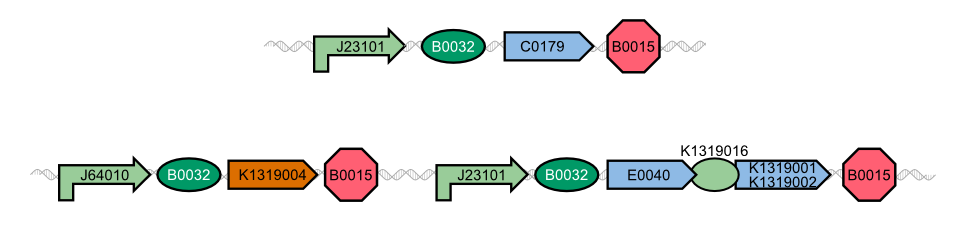
'''''Florian add diagram of I20260, K1319013 + K1319008, K1319013 + K1319008 + IPTG, K1319014 + K1319008, K1319014 + K1319008 + IPTG and B0015''''' | '''''Florian add diagram of I20260, K1319013 + K1319008, K1319013 + K1319008 + IPTG, K1319014 + K1319008, K1319014 + K1319008 + IPTG and B0015''''' | ||
| - | + | <center> | |
| + | {{Team:Aachen/Figure|https://2014.igem.org/File:Aachen 16-10-14 Graph2iFG.PNG|Comparison of K1319013 + K1319008, K1319014 + K1319008, I20260 (positive control) and B0015 (negative control)|subtitle=Fluorescence was normalized by dividing by the optical density.|width=700px}} | ||
| + | </center> | ||
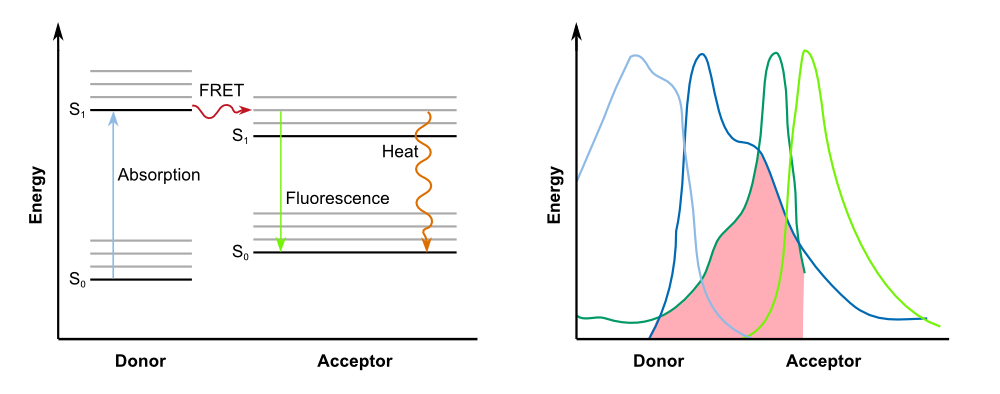
The negative control B0015 does not exhibit any significant fluuorescence as expected. The positive control I20260 shows a steady increase in fluorescence for the first X hours. After that the fluorescence stays constant due to the end of the exponential growth phase and the cells becoming stationary and not producing any more GFP. The production is also indpendent od the induction with IPTG as it is expected. | The negative control B0015 does not exhibit any significant fluuorescence as expected. The positive control I20260 shows a steady increase in fluorescence for the first X hours. After that the fluorescence stays constant due to the end of the exponential growth phase and the cells becoming stationary and not producing any more GFP. The production is also indpendent od the induction with IPTG as it is expected. | ||
| Line 182: | Line 184: | ||
Therefore K731520 and the same double plasmid construct that was described earlier (K1319013 + K1319008 and K1319014 + K1319008) were cultivated in ''E. coli'' BL21(DE3) and fluorescence and OD were measured. Once again the fluorescence was adjusted for the OD to show a relative fluorescence on a cell per cell basis. Also it was especially looked at the difference between the induced and not induced state. This difference (fluorescence quotient) gives a better indicator for a system which is used as a sensor because the difference between an ''on'' and ''off'' state is more important for a clear and unmistakable signal compared to the overall fluorescence. Hence the OD adjusted fluorescence quotient for both double plasmid constructs (K1319013 + K1319008 and K1319014 + K1319008) and K731520 was obtained and plotted in the following graphic. | Therefore K731520 and the same double plasmid construct that was described earlier (K1319013 + K1319008 and K1319014 + K1319008) were cultivated in ''E. coli'' BL21(DE3) and fluorescence and OD were measured. Once again the fluorescence was adjusted for the OD to show a relative fluorescence on a cell per cell basis. Also it was especially looked at the difference between the induced and not induced state. This difference (fluorescence quotient) gives a better indicator for a system which is used as a sensor because the difference between an ''on'' and ''off'' state is more important for a clear and unmistakable signal compared to the overall fluorescence. Hence the OD adjusted fluorescence quotient for both double plasmid constructs (K1319013 + K1319008 and K1319014 + K1319008) and K731520 was obtained and plotted in the following graphic. | ||
| - | + | <center> | |
| + | {{Team:Aachen/Figure|https://2014.igem.org/File:Aachen_16-10-14_GraphQuotient_iFG.PNG|Comparison of K1319013 + K1319008, K1319014 + K1319008 and K731520|subtitle=Fluorescence was normalized by dividing by the optical density. The fluorescence of induced cells was additionally divided by the fluorescence of not induced cells.|width=700px}} | ||
| + | </center> | ||
The graphic clearly shows the faster kinetic of the cut GFP-REACh fusion protein compared to a standard GFP expression. Both fluorescence signals of the double plasmid constructs achieve a higher difference in fluorescence signal netween induced and non induced state as well as at a faster rate. This proves the earlier made hypothesis of the kinetic of the GFP-REACh fusion protein combined with a TEV protease. | The graphic clearly shows the faster kinetic of the cut GFP-REACh fusion protein compared to a standard GFP expression. Both fluorescence signals of the double plasmid constructs achieve a higher difference in fluorescence signal netween induced and non induced state as well as at a faster rate. This proves the earlier made hypothesis of the kinetic of the GFP-REACh fusion protein combined with a TEV protease. | ||
Revision as of 09:30, 16 October 2014
|
|
|
|
|
|
|
|
 "
"