May
1st
- gel with M - full -full - REACh1 SOE3.2 - REACH2 SOE3.2 - M
- → 120 V, 30 min
- → cut out the bands
5th
- made chemical competent DH5α and BL21 cells
8th
- efficiency of our competent cells was tested
- → BL21: 6.6 x 104
- → DH5α: 2.59 x 107
- SOE-PCR step 2 like on 30.04. with the template of SOE1 from 30.04. was re-done
- SOE2 product was run on a gel for checking (5 µL)
- → restriction, (dephosphorylation of vector)
- → purification on a gel with high pure kit
14th
- made some LB agar plates with chloramphenicol and some with ampicillin
- REACh2 purification on 1.2 % agarose gel
- subsequent purification of the 778 bp fragment with High Pure PCR Product Purification Kit
19th
- transformation of K131026 in DH5α and NEB
- transformation of K731520 in DH5α
20th
- made master plates on chloramphenicol (cam) → at least 6 clones on each plate
- prepared 2x 5 mL LB + cam
- made sterile 50 % glycerol
21th
- Cryo stocks of clones 1 & 2 of each BioBrick/ host were prepared
- 15 mL cultures in 250 mL flasks, inoculated with 1.5 mL preculture (3 cultures A B C from clones 1; 1 + 2; 2)
- 250 µl Chloramphilicol from a 35 mg/mL stock was added
- The cultures were grown until OD600= 0.6, then one was induced with IPTG
| time | info/ OD
|
| 11:10 | A: inoculated | B: inoculated | C: inoculated
|
| 11:38 | A: 0.482 | B: 0.464 | C: 0.466
|
| 12:28 | A: 0.586; induced with 0.1 mM IPTG | B: 0.576 | C: 0.568
|
| 14:11 | A: 0.93 | B: 0.91 | C: 0.942
|
→ a negative control without plasmid was left out
23rd
- transformation of some BioBricks
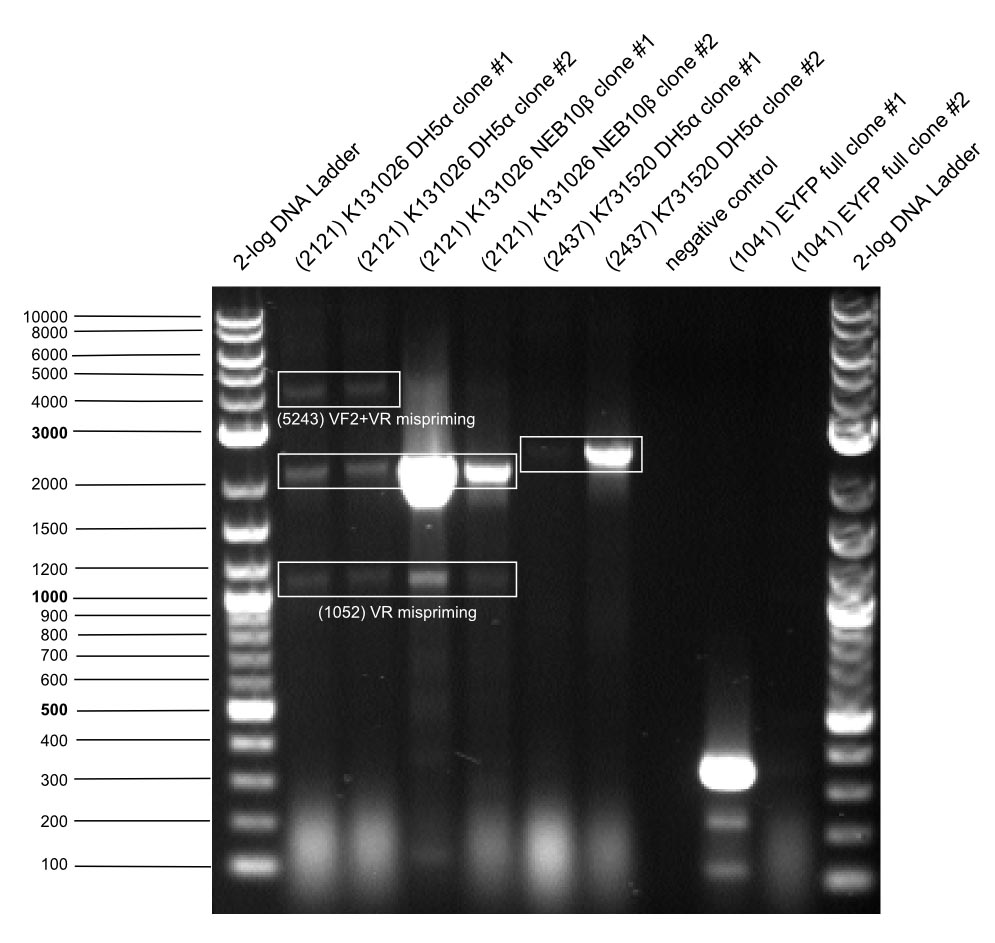
- colony PCR (s. picture below)
- → K131026: 1807 bp
- → K731520: 2123 bp
- → full length EYFP: 778 bp
|
 "
"