Achievements
Due to the generous support of Sophia Böcker and Prof. Dr. Elling of the Helmholtz Institute for Biomedical Engineering in Aachen, we got access to a pET17-derived expression plasmid for a His- and SNAP-tagged YFP-galectin-3 fusion protein. We transformed the fusion protein into E. coli Rosetta cells and conducted a batch fermentation to obtain large amounts of protein.
With the help of David Schönauer and Alan Mertens from the RWTH Aachen Institute of Biotechnolgy we then purified the fusion protein using FPLC.
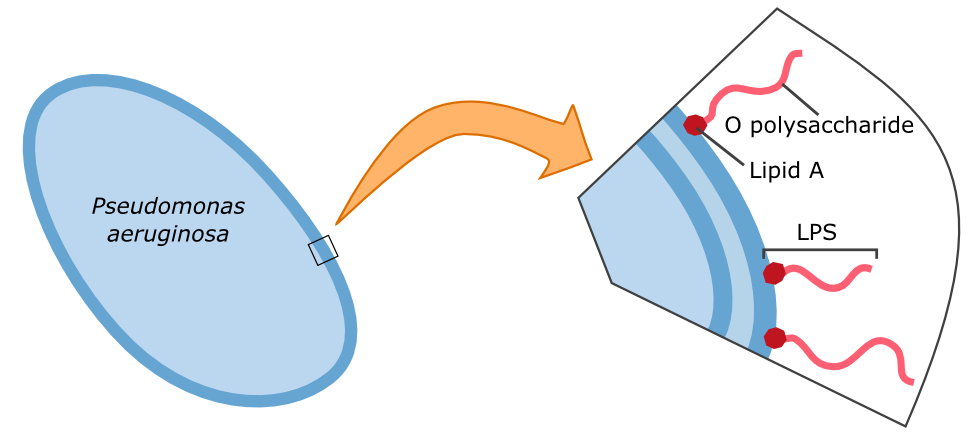
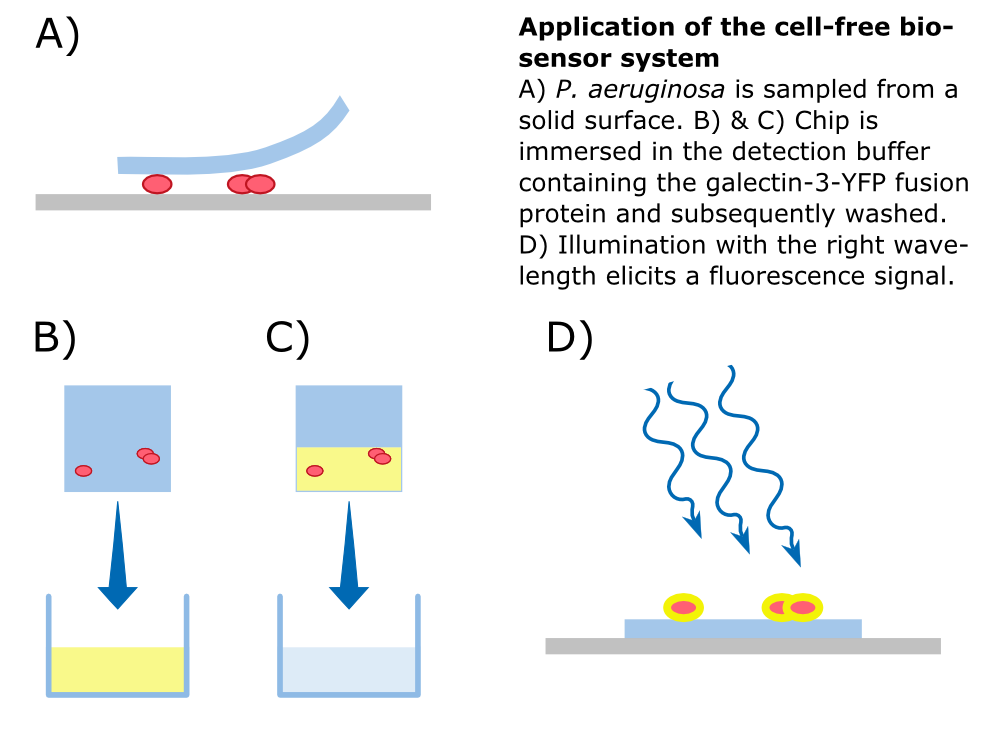
Subsequently, we aimed to test the binding of the Gal-3 fusion protein to the LPS of P. aeruginosa as shown previously [1]. Apparently, because of insufficient sensitivity of the used fluorescence microscope, this could not be confirmed and would require further experiments, idealy using other detection methods.
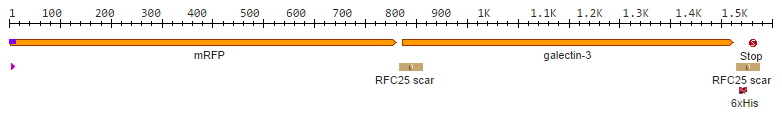
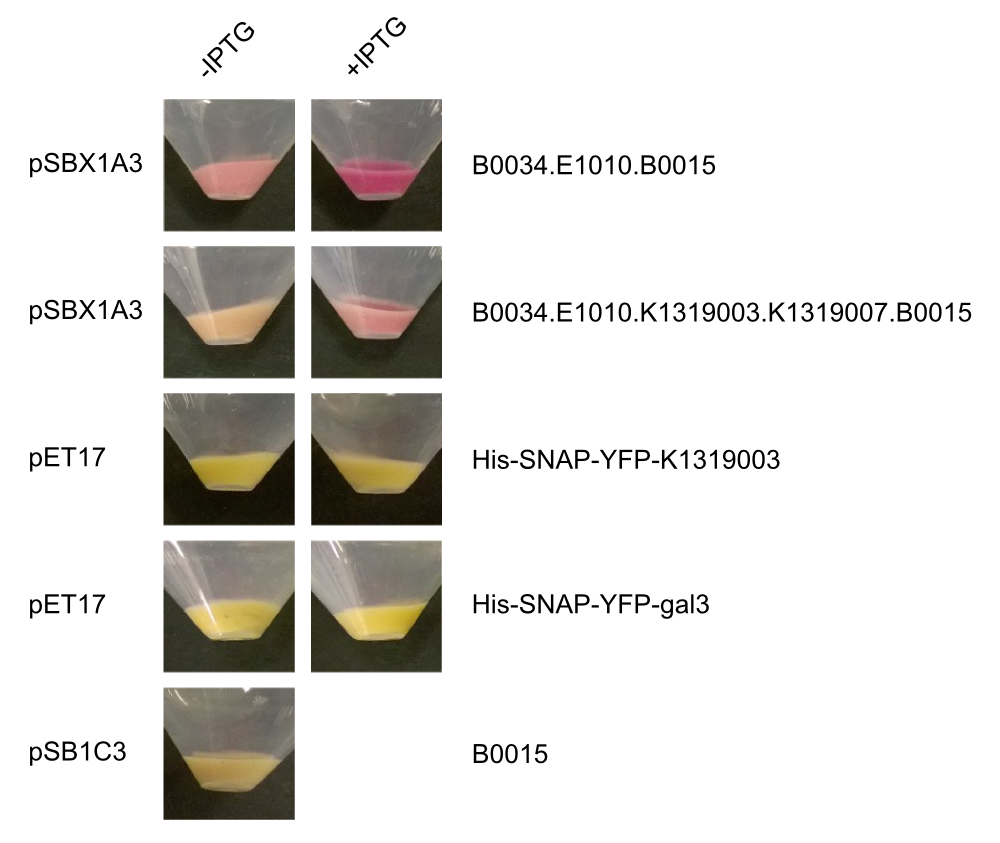
After we received the collection of pSBX-expression vectors from Team Heidelberg, we used Gibson assembly to make K1319020 from K1319003 and pSBX1A3, which is the translational unit for an mRFP-Gal3 fusion protein with a C-terminal 6xHis tag:
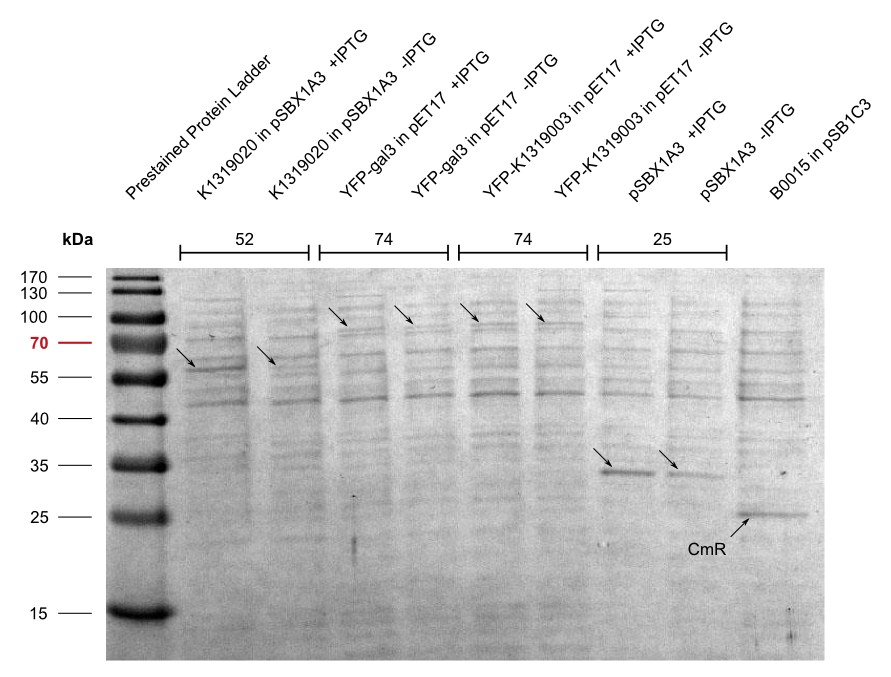
In addition, we cloned our BioBrick K1319003 into the pET17 expression vector and expressed all combinations of fusion proteins in E. coli BL21(DE3). An SDS-PAGE showed that all fusion proteins were fully translated:
References
[1] Kupper CE, Böcker S, Liu H, et al. Fluorescent SNAP-tag galectin fusion proteins as novel tools in glycobiology. Curr Pharm Des. 2013;19(30):5457-67. Available at: http://www.ncbi.nlm.nih.gov/pubmed/23431989.
|
 "
"