Team:Aachen/Project/2D Biosensor
From 2014.igem.org
(→Equipment and medium selection) |
(→Development & Optimization) |
||
| Line 143: | Line 143: | ||
=== Optimal agarose concentration for sensor chip manufacturing === | === Optimal agarose concentration for sensor chip manufacturing === | ||
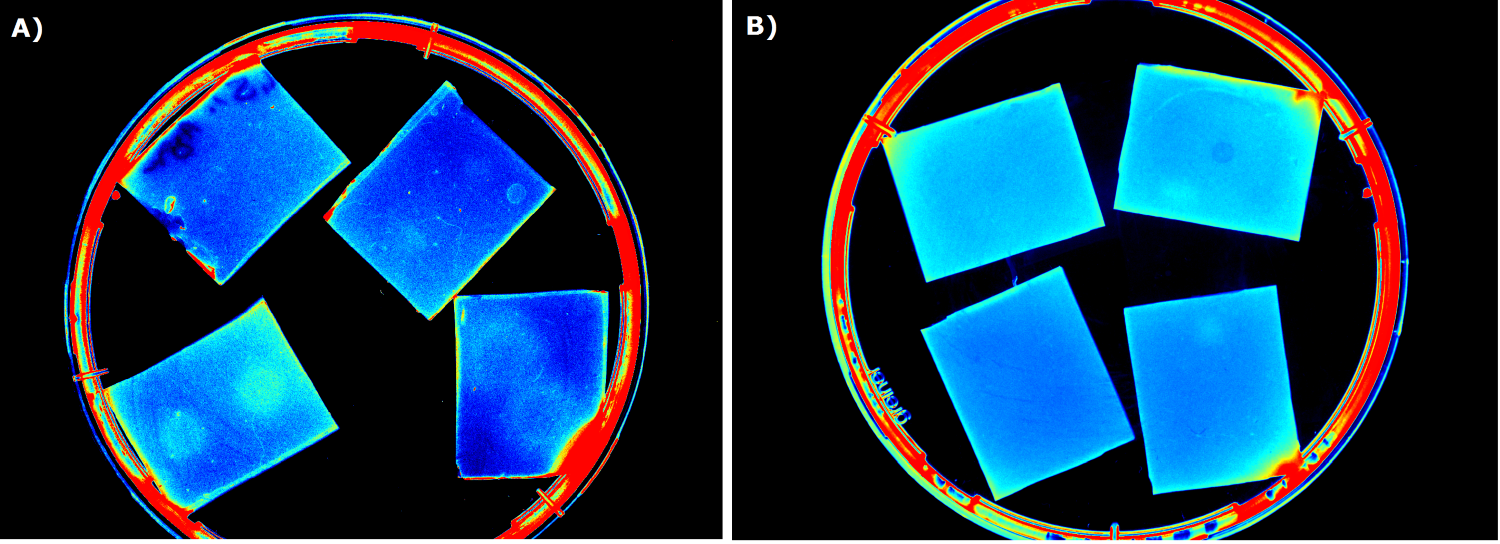
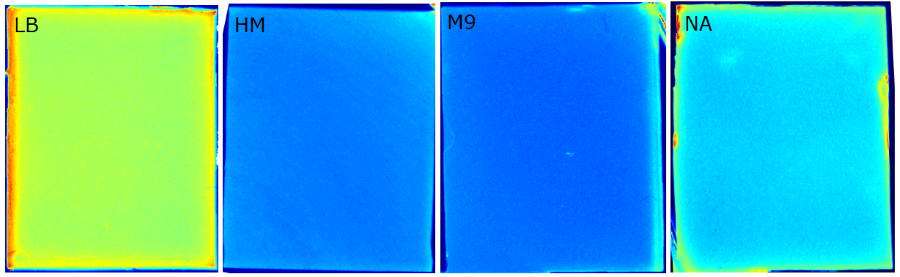
For the sensor chip manufacturing, agarose was preferred over agar, because of a uniform linkage that resulted in a better chip homogeneity. In addition, agarose reduced diffusion of the inducer molecules through the chip. A reduced diffusion is vital to observe distinct fluorescent spots on the sensor chips and thus further optimzation of our 2D biosensor was done using agarose-based chips. | For the sensor chip manufacturing, agarose was preferred over agar, because of a uniform linkage that resulted in a better chip homogeneity. In addition, agarose reduced diffusion of the inducer molecules through the chip. A reduced diffusion is vital to observe distinct fluorescent spots on the sensor chips and thus further optimzation of our 2D biosensor was done using agarose-based chips. | ||
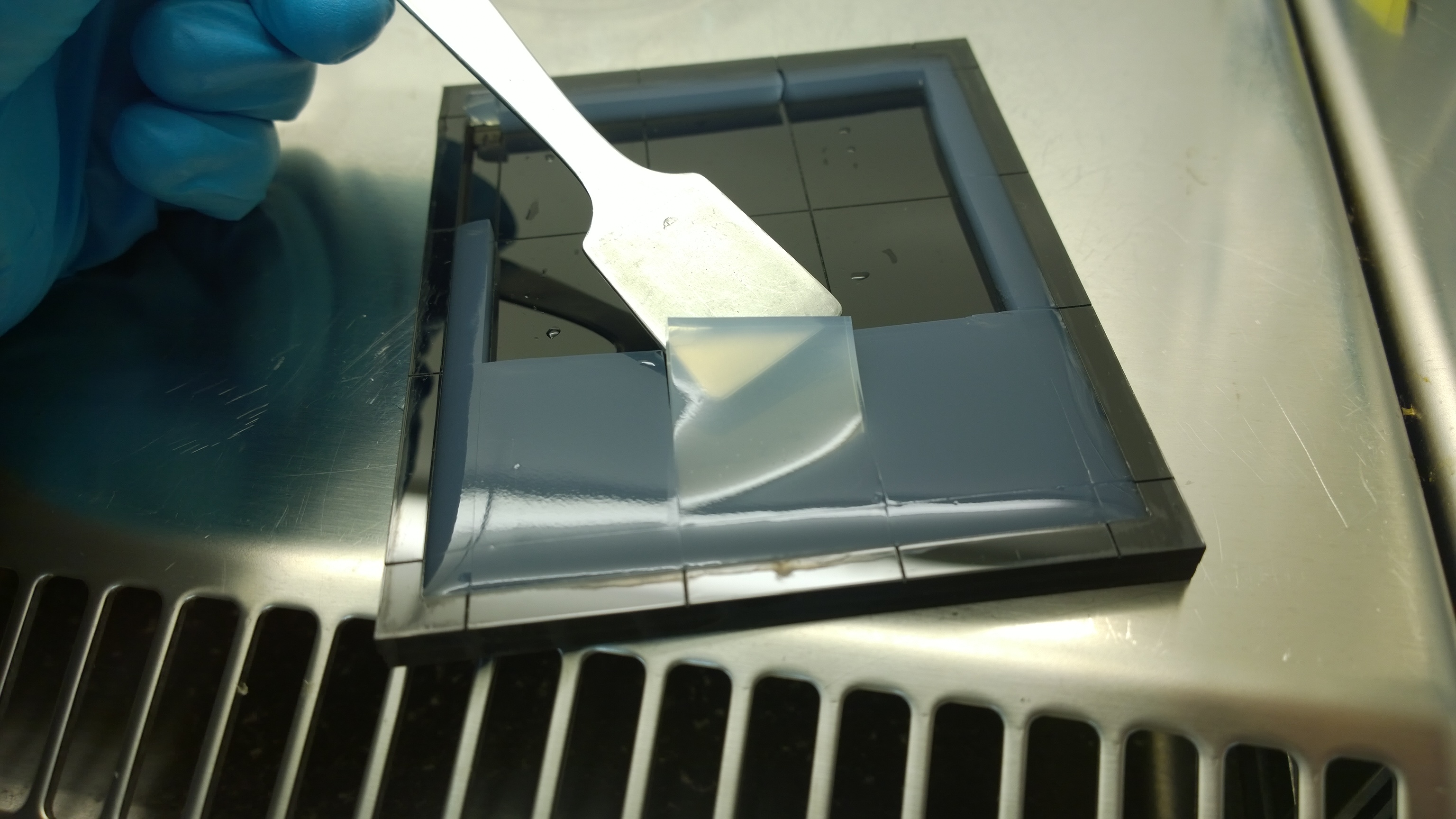
| + | {{Team:Aachen/FigureFloat|Aachen_Final_chipform.jpg|title=The finalized chip mold|subtitle=An open casting mold was found to be optimal for sensor chip manufacturing, because this approach was fast, easy to handle and generated a reproducible chip quality. In addition nine sensor chips could be produced simultaneously.|left|width=500px}} | ||
=== Optimal chip configuration === | === Optimal chip configuration === | ||
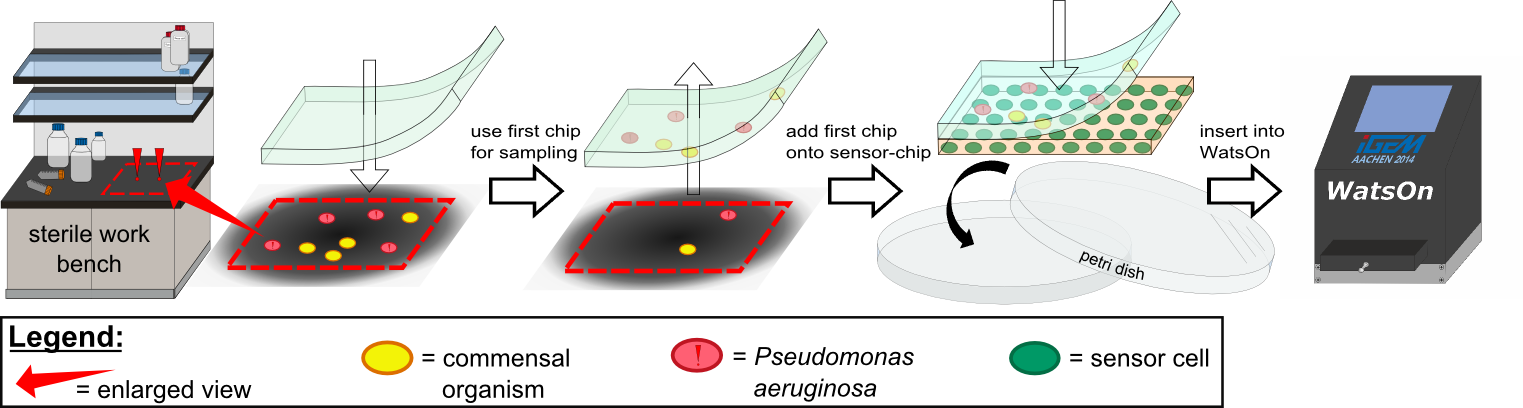
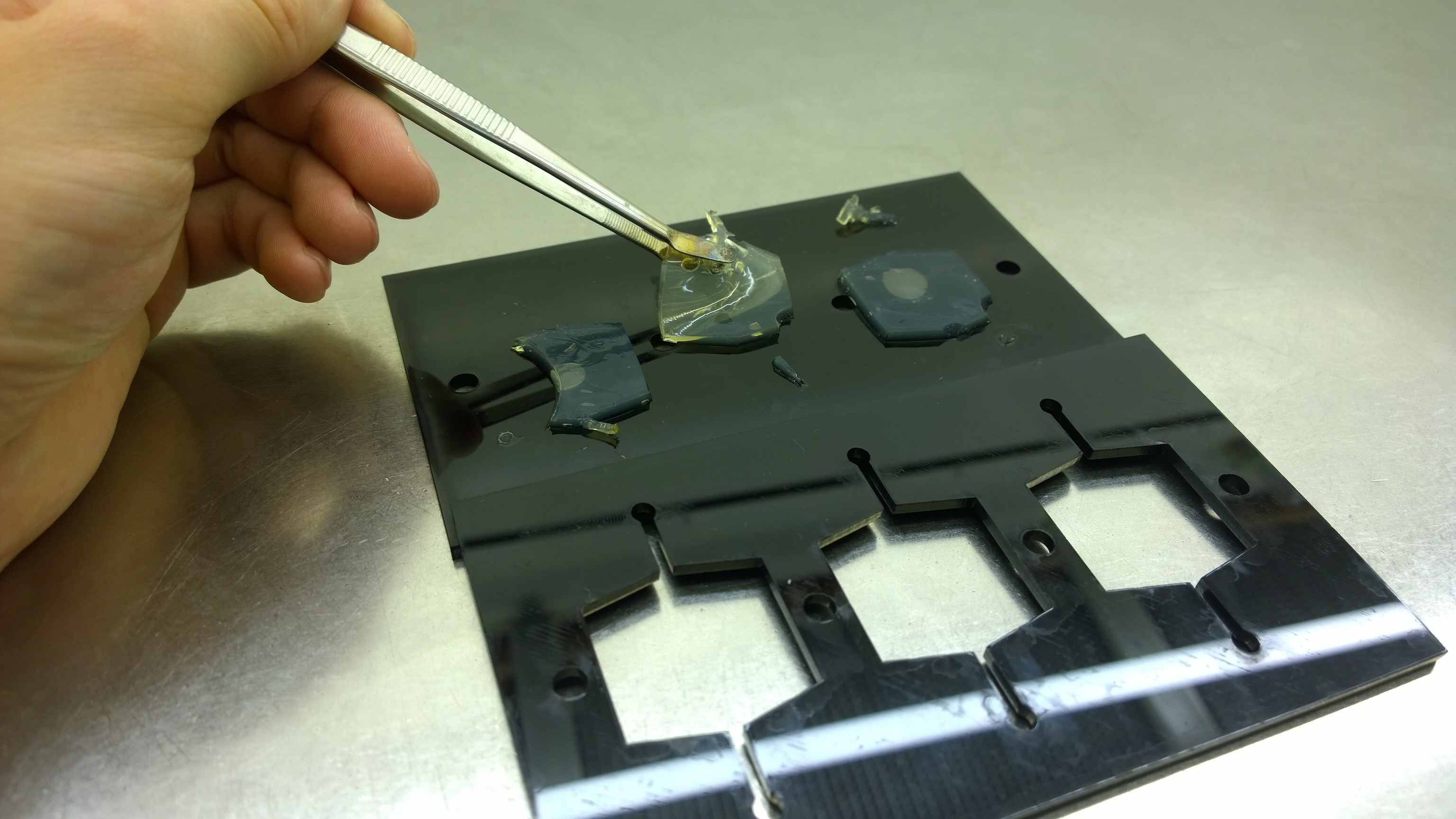
Several approaches were tested for the production of agarose-based sensor chips with reproducible quality. The first approach was to cast every sensor chip individually. To achieve a plain chip surface (a requisite for high quality images), we casted the sensor chips between two microscope slides in an initial attempt. However, this approach was rejected, because the agar was too liquid and leaked from the microscope slides. In the second approach, we prepared a closed mold into which liquid agar was injected with a pipette, but we encountered a high number of bubbles in the resulting chips. Bubbles in the sensor chips interfered with fluorescence evaluation. Finally, we used an open mold into which the agar was poured right after mixing with the sensor cells. Chips were cut out along precast indentations in the casting mold after the agar solidified. An advantage of the open mold was the ability to simultaneously produce nine sensor chips while the surface tension of the liquid agar ensured a plane chip surface. | Several approaches were tested for the production of agarose-based sensor chips with reproducible quality. The first approach was to cast every sensor chip individually. To achieve a plain chip surface (a requisite for high quality images), we casted the sensor chips between two microscope slides in an initial attempt. However, this approach was rejected, because the agar was too liquid and leaked from the microscope slides. In the second approach, we prepared a closed mold into which liquid agar was injected with a pipette, but we encountered a high number of bubbles in the resulting chips. Bubbles in the sensor chips interfered with fluorescence evaluation. Finally, we used an open mold into which the agar was poured right after mixing with the sensor cells. Chips were cut out along precast indentations in the casting mold after the agar solidified. An advantage of the open mold was the ability to simultaneously produce nine sensor chips while the surface tension of the liquid agar ensured a plane chip surface. | ||
| - | |||
| - | |||
=== Induction of the sensor chips === | === Induction of the sensor chips === | ||
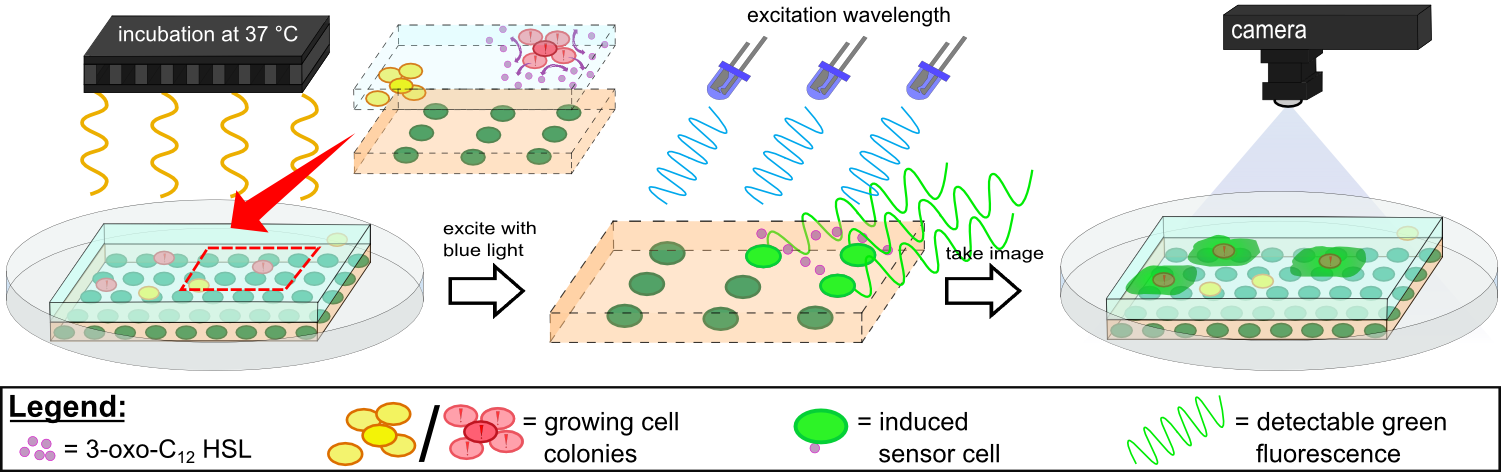
For initial testing of our molecular constructs, we simulated the presence of ''P. aeruginosa'' using IPTG or 3-oxo-C12 HSL. However, for induction a minimal volume is required as our initial experiments showed that diffusion of the inducers through the chip hindered the formation of distinct spots on the chips. An optimal and low volume of 0.2 µl was chosen for induction. Sensor cells based on ''E. coli'' BL21, which incorporated the [https://2014.igem.org/Team:Aachen/Parts#partsK1319042 K1319042] construct were able to detect IPTG concentrations down to 1 mM (0.2 µl), and as well for the sensor cells based on ''E. coli'' BL21 which incorporated the REACh constructs. Sensor cells based on ''E. coli'' BL21, which incorporated the [http://parts.igem.org/Part:BBa_K131026 K131026] construct were able to detect HSL concentrations down to 500 µg/ml (0.2 µl). Furthermore, detection of growing ''P. aeruginosa'' cells based on secreted HSLs was possible using the [http://parts.igem.org/Part:BBa_K131026 K131026] construct. The experiments conducted during induction of our sensor chips are described in more detail in the [https://2014.igem.org/Team:Aachen/Project/2D_Biosensor#biosensorachievements Achievements] section. | For initial testing of our molecular constructs, we simulated the presence of ''P. aeruginosa'' using IPTG or 3-oxo-C12 HSL. However, for induction a minimal volume is required as our initial experiments showed that diffusion of the inducers through the chip hindered the formation of distinct spots on the chips. An optimal and low volume of 0.2 µl was chosen for induction. Sensor cells based on ''E. coli'' BL21, which incorporated the [https://2014.igem.org/Team:Aachen/Parts#partsK1319042 K1319042] construct were able to detect IPTG concentrations down to 1 mM (0.2 µl), and as well for the sensor cells based on ''E. coli'' BL21 which incorporated the REACh constructs. Sensor cells based on ''E. coli'' BL21, which incorporated the [http://parts.igem.org/Part:BBa_K131026 K131026] construct were able to detect HSL concentrations down to 500 µg/ml (0.2 µl). Furthermore, detection of growing ''P. aeruginosa'' cells based on secreted HSLs was possible using the [http://parts.igem.org/Part:BBa_K131026 K131026] construct. The experiments conducted during induction of our sensor chips are described in more detail in the [https://2014.igem.org/Team:Aachen/Project/2D_Biosensor#biosensorachievements Achievements] section. | ||
Revision as of 21:01, 17 October 2014
|
|
|
|
|
|
 "
"