Team:Aachen/Project/2D Biosensor
From 2014.igem.org
(→Equipment and medium selection) |
(→Equipment and medium selection) |
||
| Line 122: | Line 122: | ||
Our first approach (before developing our own device) was to use the Molecular Imager® Gel Doc™ XR+ from BIO-RAD in our lab to detect fluorescence, where it uses UV and white light illuminators. However, only two different filters were available for the excitation light wavelength, which resulted in very limited possibilities for the excitation of fluorescent molecules. For example, it was possible to detect the expression of iLOV in our sensor chips, but in contrast the detection of GFP was not possible. Hence, the '''Gel Doc™ was not suitable for our project'''. | Our first approach (before developing our own device) was to use the Molecular Imager® Gel Doc™ XR+ from BIO-RAD in our lab to detect fluorescence, where it uses UV and white light illuminators. However, only two different filters were available for the excitation light wavelength, which resulted in very limited possibilities for the excitation of fluorescent molecules. For example, it was possible to detect the expression of iLOV in our sensor chips, but in contrast the detection of GFP was not possible. Hence, the '''Gel Doc™ was not suitable for our project'''. | ||
| - | |||
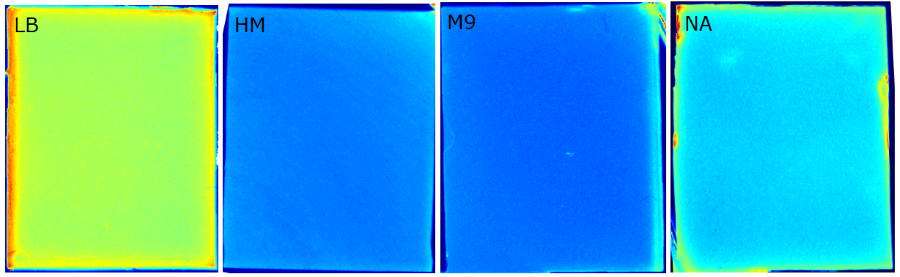
{{Team:Aachen/FigureFloat|Aachen_Chip_medium_geldoc.png|title=Differend medium in the Gel Doc™|subtitle=complex media exhibited high background fluorescence while less back-ground fluorescence was observed with the minimal media (HM, M9, NA).|right|width=500px}} | {{Team:Aachen/FigureFloat|Aachen_Chip_medium_geldoc.png|title=Differend medium in the Gel Doc™|subtitle=complex media exhibited high background fluorescence while less back-ground fluorescence was observed with the minimal media (HM, M9, NA).|right|width=500px}} | ||
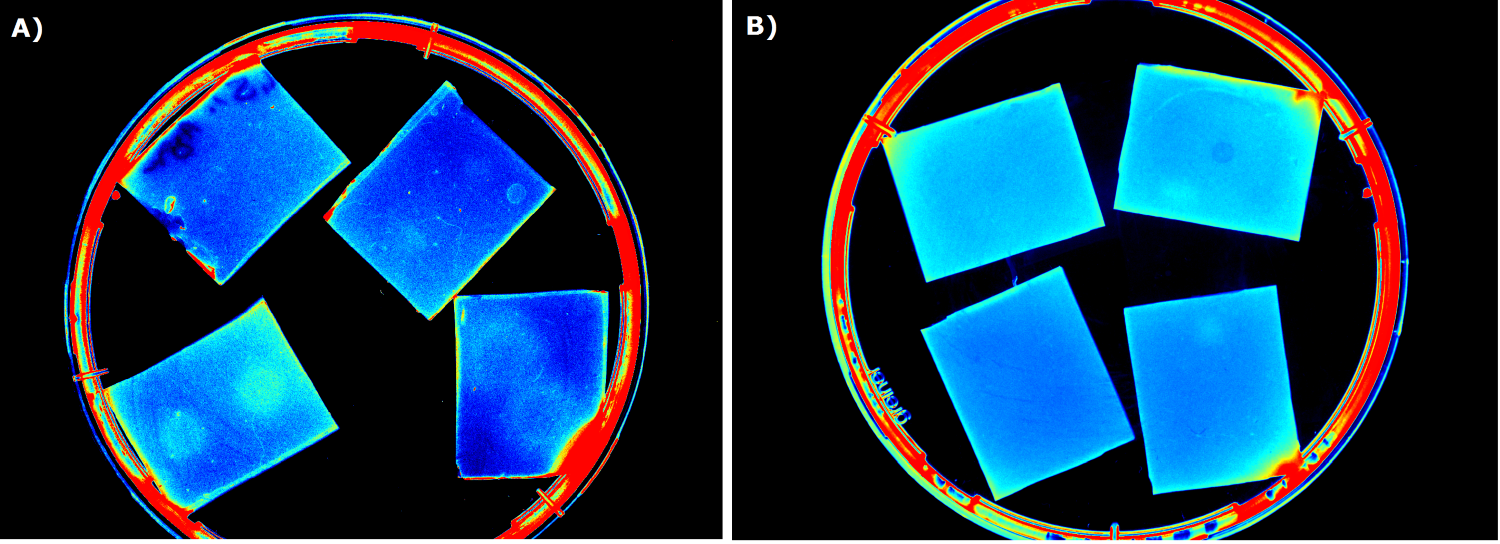
{{Team:Aachen/FigureFloat|Aachen_5days_K131026_neb_tb_1,5h.jpg |title=Testing our chips' shelf-life|subtitle= [http://parts.igem.org/Part:BBa_K131026 K131026] in NEB induced after 5 days of storage at 4°C. The right chip was induced with 0.2 µL of 500 µg/mL HSL, and the image was taken after 1.5 h.|left|width=500px}} | {{Team:Aachen/FigureFloat|Aachen_5days_K131026_neb_tb_1,5h.jpg |title=Testing our chips' shelf-life|subtitle= [http://parts.igem.org/Part:BBa_K131026 K131026] in NEB induced after 5 days of storage at 4°C. The right chip was induced with 0.2 µL of 500 µg/mL HSL, and the image was taken after 1.5 h.|left|width=500px}} | ||
| - | <br>We tested different media (i.e. LB, M9, NA, HM and TB) for the preparation of our sensor chips. The details of media composition can be found in the [https://2014.igem.org/Team:Aachen/Notebook/Protocols Protocols] section. We screened for an optimized media composition that results in minimal background fluorescence and supports cell growth. The resuts of the analysis are presented in the table below. Because of the reduced fluorescence compared to TB medium when using ''WatsOn'' for sensor chip evaluation and because of sufficient cultivation conditions for our ''Cellocks'' '''LB medium was chosen over TB medium for sensor hip manufacturing'''. | + | <br> We tested different media (i.e. LB, M9, NA, HM and TB) for the preparation of our sensor chips. The details of media composition can be found in the [https://2014.igem.org/Team:Aachen/Notebook/Protocols Protocols] section. We screened for an optimized media composition that results in minimal background fluorescence and supports cell growth. The resuts of the analysis are presented in the table below. Because of the reduced fluorescence compared to TB medium when using ''WatsOn'' for sensor chip evaluation and because of sufficient cultivation conditions for our ''Cellocks'' '''LB medium was chosen over TB medium for sensor hip manufacturing'''. |
<center> | <center> | ||
| Line 141: | Line 140: | ||
Experiments were conducted to test '''long-time storage''' of the sensor chips at low temperature and by the addition of | Experiments were conducted to test '''long-time storage''' of the sensor chips at low temperature and by the addition of | ||
glycerol. Storage at -20°C resulted in the loss of our sensor cells. Adding 5-10% (v/v) glycerol ensured survival of the sensor cells, but resulted in an expression stop of fluorescence proteins. Hence, we concluded that long time storage of the sensor chips is not possible under the tested conditions. However, it is possible to store 'ready-to-use' sensor chips for 2 days at 4°C when using LB medium and storage for 5 days was possible with chips made from TB medium. | glycerol. Storage at -20°C resulted in the loss of our sensor cells. Adding 5-10% (v/v) glycerol ensured survival of the sensor cells, but resulted in an expression stop of fluorescence proteins. Hence, we concluded that long time storage of the sensor chips is not possible under the tested conditions. However, it is possible to store 'ready-to-use' sensor chips for 2 days at 4°C when using LB medium and storage for 5 days was possible with chips made from TB medium. | ||
| + | |||
| + | <!--Regarding the medium used for our sensor chips, LB medium showed a high background fluorescence when exposed to UV light in the Gel Doc. Surprisingly, the background fluorescence resulting from the LB medium was too high to detect a signal emitted by our sensor cells. Hence, minimal media (NA, M9, Hartman (HM)) was used to minimize background fluorescence, but this approach resulted in less to no growth of our sensor cells. In our device ''WatsOn'', optimized wavelengths of 450 nm and 480 nm were used for excitation of iLOV and GFP, respectively. When exposed to either excitation wavelength TB medium, which is basically an improved LB medium and highly supports cell growth, showed strong background fluorescence in our own device. High background fluorescence was also observed for TB medum when using the Gel Doc. In contrast to the Gel Doc LB medium showed minimal fluorescence in our device ''WatsOn'' and no difficulties in cultivation of our ''Cellocks'' were observed. Because of the reduced fluorescence compared to TB medium when using ''Watson'' for sensor chip evaluation and because of sufficient cultivation conditions for our 'Cellocks'' LB medium was chosen over TB mediium for sensor chip manufacturing. --> | ||
=== Optimal agarose concentration for the sensor chip === | === Optimal agarose concentration for the sensor chip === | ||
Revision as of 18:03, 17 October 2014
|
|
|
|
|
|
 "
"