Team:SUSTC-Shenzhen/Notebook/HeLaCell/Construct-Cellline
From 2014.igem.org
(Add picture) |
|||
| Line 225: | Line 225: | ||
{{SUSTC-Image|wiki/images/6/66/SUSTC-Shenzhen-3DayAfterTransefation-8.17G2.tif| G2 3days}} | {{SUSTC-Image|wiki/images/6/66/SUSTC-Shenzhen-3DayAfterTransefation-8.17G2.tif| G2 3days}} | ||
| - | Figure . The fluorescent picture of G2. Rate of fluorescent and intensity is much lower comparing with G1. | + | Figure . The fluorescent picture of G2. Rate of fluorescent and intensity is much lower comparing with G1. |
| - | {{SUSTC-Image|wiki/images/d/d2/SUSTC-Shenzhen-3DayAfterTransefation-8.17G3.tif|G3 3days}} | + | {{SUSTC-Image|wiki/images/d/d2/SUSTC-Shenzhen-3DayAfterTransefation-8.17G3.tif| G3 3days}} |
| - | Figure . The fluorescent picture of G3. G3 has transfected with plasmid expressing Cas9 protein and GFP. | + | Figure . The fluorescent picture of G3. G3 has transfected with plasmid expressing Cas9 protein and GFP.About 1/3-1/5 cells has been killed. |
| - | {{SUSTC-Image|wiki/images/d/d2/SUSTC-Shenzhen-3DayAfterTransefation-8.17G4.tif|G4 3days}} | + | {{SUSTC-Image|wiki/images/d/d2/SUSTC-Shenzhen-3DayAfterTransefation-8.17G4.tif| G4 3days}} |
Figure . The fluorescent picture of G4. Same composition of plasmid as G3, but with lower usage of plasmid pBx-083 lower rate of transfection and lower intensity of fluorescencs. | Figure . The fluorescent picture of G4. Same composition of plasmid as G3, but with lower usage of plasmid pBx-083 lower rate of transfection and lower intensity of fluorescencs. | ||
| - | {{SUSTC-Image|wiki/images/a/aa/SUSTC-Shenzhen-3DayAfterTransefation-8.17G5.tif|G5}} | + | {{SUSTC-Image|wiki/images/a/aa/SUSTC-Shenzhen-3DayAfterTransefation-8.17G5.tif| G5 3days}} |
Figure . The fluorescent picture of G5. No green fluorescence on living cells, only some dead cells with auto-fluorescence. | Figure . The fluorescent picture of G5. No green fluorescence on living cells, only some dead cells with auto-fluorescence. | ||
| Line 255: | Line 255: | ||
===Procedures=== | ===Procedures=== | ||
| + | |||
# Wash cells with PBS for 2 times | # Wash cells with PBS for 2 times | ||
# Add trypsin 1ml and discard to digest for 2min at 37°C. | # Add trypsin 1ml and discard to digest for 2min at 37°C. | ||
| Line 265: | Line 266: | ||
'''''The forth day after transfection''''' | '''''The forth day after transfection''''' | ||
| - | + | '''''The first day selection''''' | |
Start the screening of the transfected cells on the plate with corresponding antibiotics. | Start the screening of the transfected cells on the plate with corresponding antibiotics. | ||
| Line 296: | Line 297: | ||
'''''The fifth day after transfection''''' | '''''The fifth day after transfection''''' | ||
| + | '''''The second day selection''''' | ||
Observe the cells on the petri dish with fluorescence microscope. It is easy to find many living with green fluorescence in the pate, and the fluorescence of groups 1&3 is stronger than that of groups 2&4 moreover group 1&3 have much more cells with fluorescence than 2&4. | Observe the cells on the petri dish with fluorescence microscope. It is easy to find many living with green fluorescence in the pate, and the fluorescence of groups 1&3 is stronger than that of groups 2&4 moreover group 1&3 have much more cells with fluorescence than 2&4. | ||
| Line 350: | Line 352: | ||
Cells on these plate has already grows into small monoclones. And some of clones having no fluorescence as well as resistance have not been killed by antibiotics. Clones and clones separate widely normally 2-3 clones per view. | Cells on these plate has already grows into small monoclones. And some of clones having no fluorescence as well as resistance have not been killed by antibiotics. Clones and clones separate widely normally 2-3 clones per view. | ||
| + | |||
| + | ===Result=== | ||
| + | Plates to construct has a much higher concentration, esay to find a view with many cells. Group 1,3 has more cells with fluorescencs and more intense than group 2,4. | ||
| + | |||
| + | //TODO{ | ||
| + | ==Fluorescent Observation== | ||
| + | [2014 Aug 21st 12:00am] | ||
| + | |||
| + | '''''The sixth day after transfection''''' | ||
| + | '''''The third day selection''''' | ||
| + | |||
| + | # G1-4 50,000 plate has many cells dead. Morethan half cells have been killed. | ||
| + | # Pool plate | ||
| + | ## G5: Most cells are dead, only 1~2 clones per view field at 4x objective lens. | ||
| + | ## G4: Many cells are dead, less than one clone every view field of 4x objective lens 10~20 cells per clone. | ||
| + | ## G3: Many cells are dead and suspend in medium. 5~10 clone per view field. 30 cells per clone. | ||
| + | ## G2: More cells than G4 but less than G3. | ||
| + | ## G1: More cells than G3. | ||
| + | }//TODO | ||
| + | |||
| + | ==Change medium== | ||
| + | [2014 Aug 21st 14:00pm] | ||
| + | |||
| + | All plate have been changed medium. The medium is seriously vaporated, only 3ml medium left in plate, 5ml orginally. | ||
==Seed plate for second transfection== | ==Seed plate for second transfection== | ||
| Line 355: | Line 381: | ||
'''''The seventh day after transfection''''' | '''''The seventh day after transfection''''' | ||
| + | '''''The forth day selection''''' | ||
Prepare for the second transfection. <br/> | Prepare for the second transfection. <br/> | ||
| Line 381: | Line 408: | ||
# Add 1ml PBS to every gap between well | # Add 1ml PBS to every gap between well | ||
# 1:10 dilution count the cell concentration with blood counting slide. Get the concentration: 760,000 cells/ml. 0.78ml cell suspendion + about 9ml DMEM with 10% FBS. Mix well and add 1.5ml per well, to mix well pepite the well gently. | # 1:10 dilution count the cell concentration with blood counting slide. Get the concentration: 760,000 cells/ml. 0.78ml cell suspendion + about 9ml DMEM with 10% FBS. Mix well and add 1.5ml per well, to mix well pepite the well gently. | ||
| + | |||
| + | ===Result=== | ||
| + | Cells seeded really even. | ||
//TODO{ | //TODO{ | ||
| + | ==Fluorescence Observation== | ||
| + | [2014 Aug 23rd 10:29] | ||
| + | '''''The eighth day after transfection''''' | ||
| + | '''''The fifth day selection''''' | ||
| + | |||
| + | After 5 days selection, almost every cell surviving expressing green fluorescence. Only a few cells on 10cm petri dish for monoclones do not express green fluorescence.<br> | ||
| + | When peak up monoclones to construct a stable cell-line, we will check its green fluorescence under fluorescence microscope. | ||
| + | |||
| + | ===Monoclones=== | ||
| + | {{SUSTC-Image|wiki/images/3/3a/SUSTC-Shenzhen-G1_monoclone_7days.png|G1 monoclones}} | ||
| + | Figure . Picture of G1 monoclones, almost every cells has fluorescece. | ||
| + | |||
| + | {{SUSTC-Image|wiki/images/9/9f/SUSTC-Shenzhen-G2_monoclone_7days.png|G2 monoclones}} | ||
| + | Figure . Picture of G2 monoclones, hardly to find cells expressing GFP. | ||
| + | |||
| + | {{SUSTC-Image|wiki/images/1/14/SUSTC-Shenzhen-G3_monoclone_7days.png|G3 monoclones}} | ||
| + | Figure . Picture of G3 monoclones, almost every cells has GFP. | ||
| + | |||
| + | {{SUSTC-Image|wiki/images/c/c0/SUSTC-Shenzhen-G4_monoclone_7days.png|G4 monoclones}} | ||
| + | Figure . Picture of G4 monoclones, some of cells expressing GFP. | ||
| + | |||
| + | We observed and note clones expressing clones in all clones we observed under fluorescent microscope. | ||
| + | <html><div class="table-responsive"></html> | ||
| + | {|class="table" | ||
| + | |+ style="font-size: 19px; font-weight: bold;" |Ratio of fluorescence | ||
| + | !Groups | ||
| + | !With GFP | ||
| + | !Without GFP | ||
| + | |- | ||
| + | !Group 1 | ||
| + | |11 | ||
| + | |12 | ||
| + | |- | ||
| + | !Group 2 | ||
| + | |4 | ||
| + | |11 | ||
| + | |- | ||
| + | !Group 3 | ||
| + | |10 | ||
| + | |10 | ||
| + | |- | ||
| + | !Group 4 | ||
| + | |9 | ||
| + | |11 | ||
| + | |- | ||
| + | |} | ||
| + | <html></div></html> | ||
| + | |||
| + | ===Pool cells=== | ||
| + | {{SUSTC-Image|wiki/images/0/02/SUSTC-Shenzhen-G1_pool_7days.png|G1 pool}} | ||
| + | |||
| + | Figure . | ||
| + | |||
| + | {{SUSTC-Image|wiki/images/3/3f/SUSTC-Shenzhen-G2_pool_7days.png|G2 pool}} | ||
| + | |||
| + | Figure . | ||
| + | |||
| + | {{SUSTC-Image|wiki/images/2/28/SUSTC-Shenzhen-G3_pool_7days.png|G3 pool}} | ||
| + | |||
| + | Figure . | ||
| + | |||
| + | {{SUSTC-Image|wiki/images/b/b7/SUSTC-Shenzhen-G4_pool_7days.png|G4 pool}} | ||
| + | Figure . | ||
//TODO} | //TODO} | ||
| + | ==Second transfection== | ||
| + | [2014 Aug 25th 11:00am] | ||
Revision as of 16:39, 12 October 2014
Notebook
Elements of the endeavor.
Contents |
Stably Transfected HeLa cell line construct
2014/8/15 Construct stably transfected HeLa cells!
Seed cells
[2014 Aug 14th 15:00pm]
Method
Seed HeLa cells in 6-well-plate (15wells at total), 200,000 cells per well. For tansfection
- Procedure
- Wash cells with PBS, trypsin digestion, and medium to stop digestion.
- Transfer the sell suspension in 15ml centrifuge tube and centrifuge at 800rpm, for 5 min.
- Discard the supernatant and add 2ml complete medium to re suspend the cells.
- Add 1ml PBS to every gap between well
- 1:10 dilution count the cell concentration with blood counting slide. Get the concentration: 2,800,000 cells/ml. 1.15ml cell suspendion + about 31ml DMEM with 5% FBS. Mix well and add 2ml per well, shake the plate gently.
Result
The cells plate unevenly concentrated at the center of each well.
Cell transfection
[2014 Aug 15th 11:00am]
Materials
- plasmid pBx-083 (EGFP)
- plasmid Piggybac
- plasmid Cas9+TetOn
- Lipofectamine 3000(Invitrogen)
- Opti-MEM
- HeLa cells in 6-well-plate
| Plasmid | Concentration(ng/μl) |
|---|---|
| EGFP(pBx-083) | 1641 |
| Cas9+TetOn | 2344.1 |
| Piggybac | 2896.7 |
Method
a. 3 combinations
- EGFP(pBx-083) & Piggybac(transposase)
- EGFP & Cas9 & Piggybac
- Cas9(Cas9+TetOn) & Piggybac
For the first two combination we have different ratio of EGFP to get mono-copy of EGFP in cell line.
| Group 1 | EGFP:Piggybac = 1:1 |
|---|---|
| Group 2 | EGFP:Piggybac = 1:9 |
| Group 3 | Cas9:EGFP:Piggybac = 1:1:1 |
| Group 4 | Cas9:EGFP:Piggybac = 3:17:10 |
| Group 5 | Cas9:Piggybac = 1:1 |
| Groups(3wells/group) | EGFP(μl) | Cas9(μl) | Piggybac(μl) |
|---|---|---|---|
| Group 1(3wells) | 2.29 | 0 | 1.30 |
| Group 2(3wells) | 0.46 | 0 | 2.33 |
| Group 3(3wells) | 1.49 | 1.359 | 0.612 |
| Group 4(3wells) | 0.46 | 1.81 | 0.86 |
| Group 5(3wells) | 0 | 1.60 | 1.30 |
Procedure
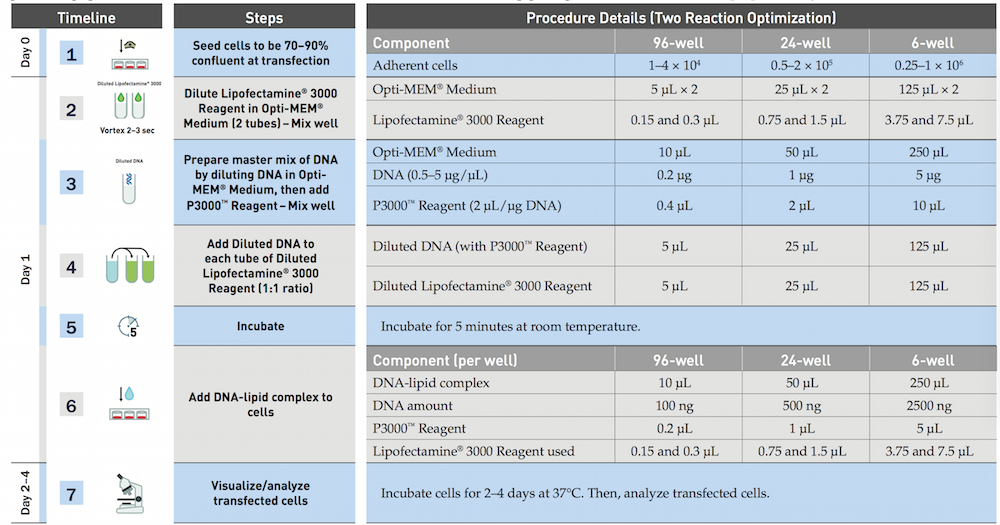
The official protocol of Lipofectamine 3000 (Invitrogen)
We use the reduced amout of Lipofectamine(lipo), and slightly improve the procedure by the experience of an RA.
- Dilute 3.75μl/well Lipofectamine 3000 reagent in 25μl/well Opti-MEM, and incubation for 5min
- Prepare master mix of 5DNA μg/well by dilutiong DNA in Opti-MEM medium 25μl/well according to the form, then add P3000 reagent.
- Add dilutetd DNA to each tube of diluted lipo 3000.
- Incubation for 10min
- Add DNA lipid complex to cell waiting for the harvest.
Laboratory note
- Add DNA to 380μL Opti-MEM medium according to the table 2 and add 15μL P3000 reagent, mix well.
- Dilute Lipofectamine 3000 Reagent in Opti-MEM Medium: (2x) 1000μL Opti-MEM Medium + 120μL Lipofectamine 3000 Reagent, mix well.
- Add 395μL Diluted Lipofectamine 3000 to Diluted DNA of each group (1:1 ratio), mix well.
- Incubate for about 10min.
- Add DNA-lipid complex (250μL per well) to cells, shake the plate. (12:00 am)
- After 10h, change the medium with the complete medium with 10% FBS and Penicillin-Streptomycin.
Microscope Observation
[2014 Aug 16th 9:00am]
The first day after transfection
We change to medium into complete medium at 10%FBS 12 hours after transfection according to protocol. And take picture under microscope.
Laboratory note
The transfected cells are observed before we change the medium.

Figure 1. Pictures are captured by phase contrast microscope. We can see clearly that some cells has suspended and became round. Strangely the G3 well 1 has a apparent amount of cells at abnormal status.
It is found that part of the cells round and suspended, and part of cells just become round. But the morphology of most of the cells are normal.
Passage cell to dish
[2014 Aug 16th 15:00pm]
The first day after transfection
Passage part of cells to the 10cm petri dish (for picking monoclonal later except G5), and the remaining cells are still seeded on the previous 24-well plates (about 500μL cell suspension per well). (15:00 pm – 18:00 pm) For every group, two dishes with 5,000 cells seeded and two dished with 50,000 cells seeded.
Procedures
- Wash cells with PBS, trypsin digestion, add medium to stop digestion
- Transfer the cell suspension into 15 mL centrifuge tube and centrifuge at 800rpm for 5 min.
- Discard the supernatant and add 2 mL complete medium to suspend the cells.
- Count the cell concentration with blood counting chamber.
- Mark the new dishes, add 5 mL medium to every dish, and add corresponding cell suspension, mix well with the pipet.
- Culture.
| Group | Counted number | Concentration (cells/mL) | Volume needed |
|---|---|---|---|
| 1 | 30 | 300,000 | 167 and 16.7 |
| 2 | 20 | 200,000 | 294 and 29.4 |
| 3 | 30 | 300,000 | 294 and 29.4 |
| 4 | 30 | 300,000 | 143 and 14.3 |
Discussion
When counting cells, some dead cells were observed, and those cells were not included. The second day morning, some of the cells on the petri dish are round and suspended. Cells were few and scattered. For dishes with 5,000 cells, only several cells can be observed in one field under 4X objective lens. For dishes with 50,000 cells, near 10-20 cells can be observed in one field under 4X objective lens.
Fluorescent Observation
[2014 Aug 18th 10:00am]
The third day after transfection
Observe the transfected cells on the plate under fluorescence microscope.

Figure . The fluorescent picture of G1 captured by microscope. of Cells has been passage to dish so the concentration is low. The rate of transefaction is pretty high, because the usage of pBx-083 expressing EGFP is high.

Figure . The fluorescent picture of G2. Rate of fluorescent and intensity is much lower comparing with G1.

Figure . The fluorescent picture of G3. G3 has transfected with plasmid expressing Cas9 protein and GFP.About 1/3-1/5 cells has been killed.

Figure . The fluorescent picture of G4. Same composition of plasmid as G3, but with lower usage of plasmid pBx-083 lower rate of transfection and lower intensity of fluorescencs.

Figure . The fluorescent picture of G5. No green fluorescence on living cells, only some dead cells with auto-fluorescence.
[2014 Aug 18th 15:00pm]
The third day after transfection
And observe the transfected cells on petri dish with 50,000 cells under fluorescence microscope. For group 1 and 3 some living cells with fluorescence can be observed but for group 2&4, it is hard to find fluorescence.
Passage cells
[2014 Aug 19th 00:00am]
The forth day after transfection
Passage cells of every Groups from wells to petri dishes. Use antibiotics to kill cells without resistance. Only those with resistance can survive, can express gene we transform both resistance gene and gene we need.
Cells left after passage to the petri dish for monoclones are passage to another petri dish to construct a pool of all kinds of cells expressing resistance.But less stable and less homogeneous than monoclones.
Procedures
- Wash cells with PBS for 2 times
- Add trypsin 1ml and discard to digest for 2min at 37°C.
- Add complete medium 2ml to end digestion, suspend cells.
- Passage cell suspension to 10cm petri dish, and 5ml complete medium mix well with pipet. Mark the dish.
Cell selection
[2014 Aug 19th 16:00pm]
The forth day after transfection The first day selection Start the screening of the transfected cells on the plate with corresponding antibiotics.
Start the selection and screening of the transfected cells on one petri dish with 50,000 cells with corresponding antibiotics.
| Group | Blasticidin(ng/μL) | Puromycin(ng/μL) |
|---|---|---|
| 1&2 | 2.8 | 0 |
| 3&4 | 2.8 | 2 |
| 5 | 0 | 2 |
Fluorescent Observation
[2014 Aug 20th 18:00pm]
The fifth day after transfection The second day selection
Observe the cells on the petri dish with fluorescence microscope. It is easy to find many living with green fluorescence in the pate, and the fluorescence of groups 1&3 is stronger than that of groups 2&4 moreover group 1&3 have much more cells with fluorescence than 2&4.
Green fluorenscence image and bright field image has been put together overlaying. Because overlay the fluorescent intensity on pictures does not represent the real fluorescent intensity. But can show the ratio of cells have fluorescence.
Pool cells
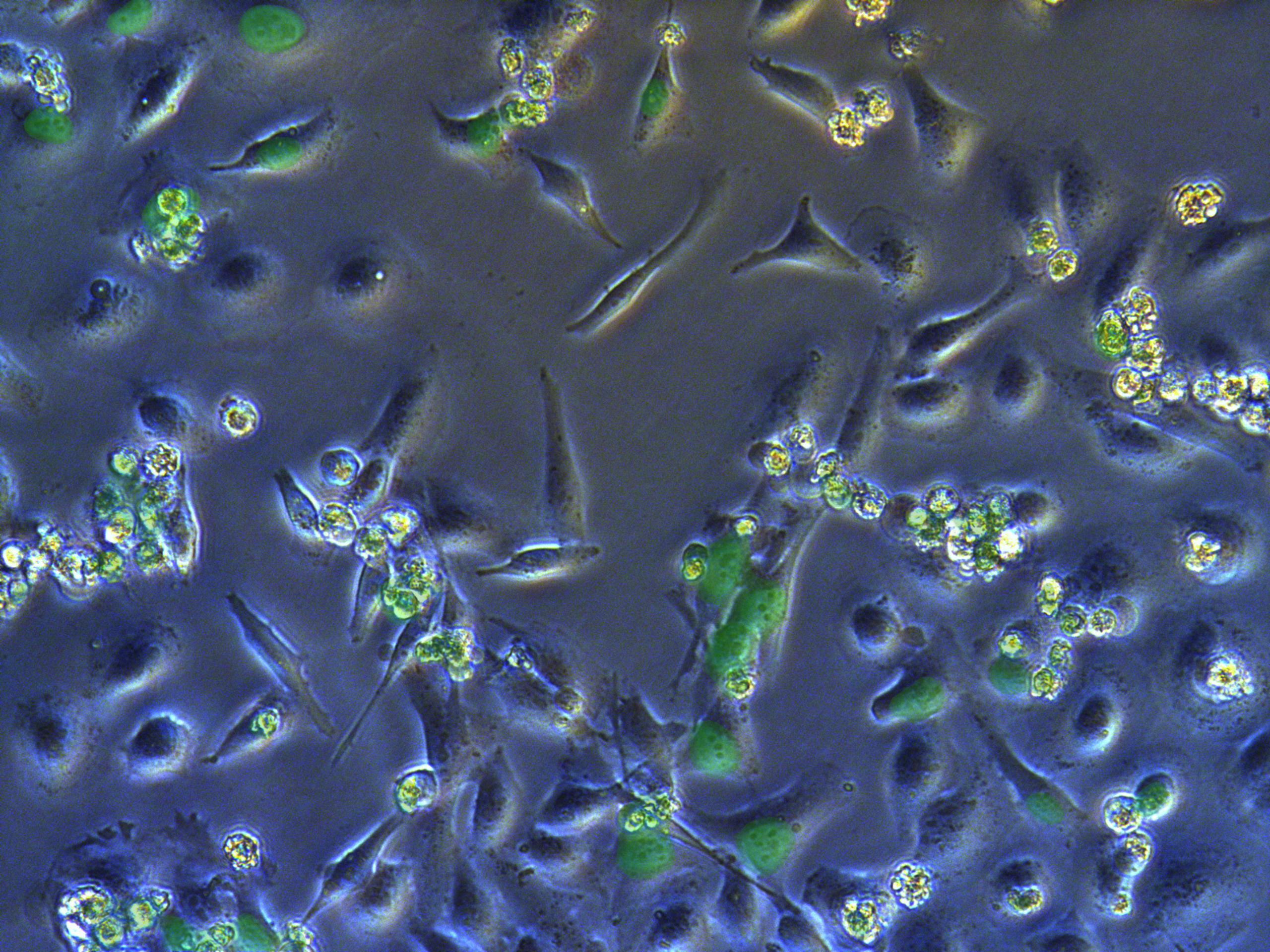
Figure . G1 pool cells on petri dish.
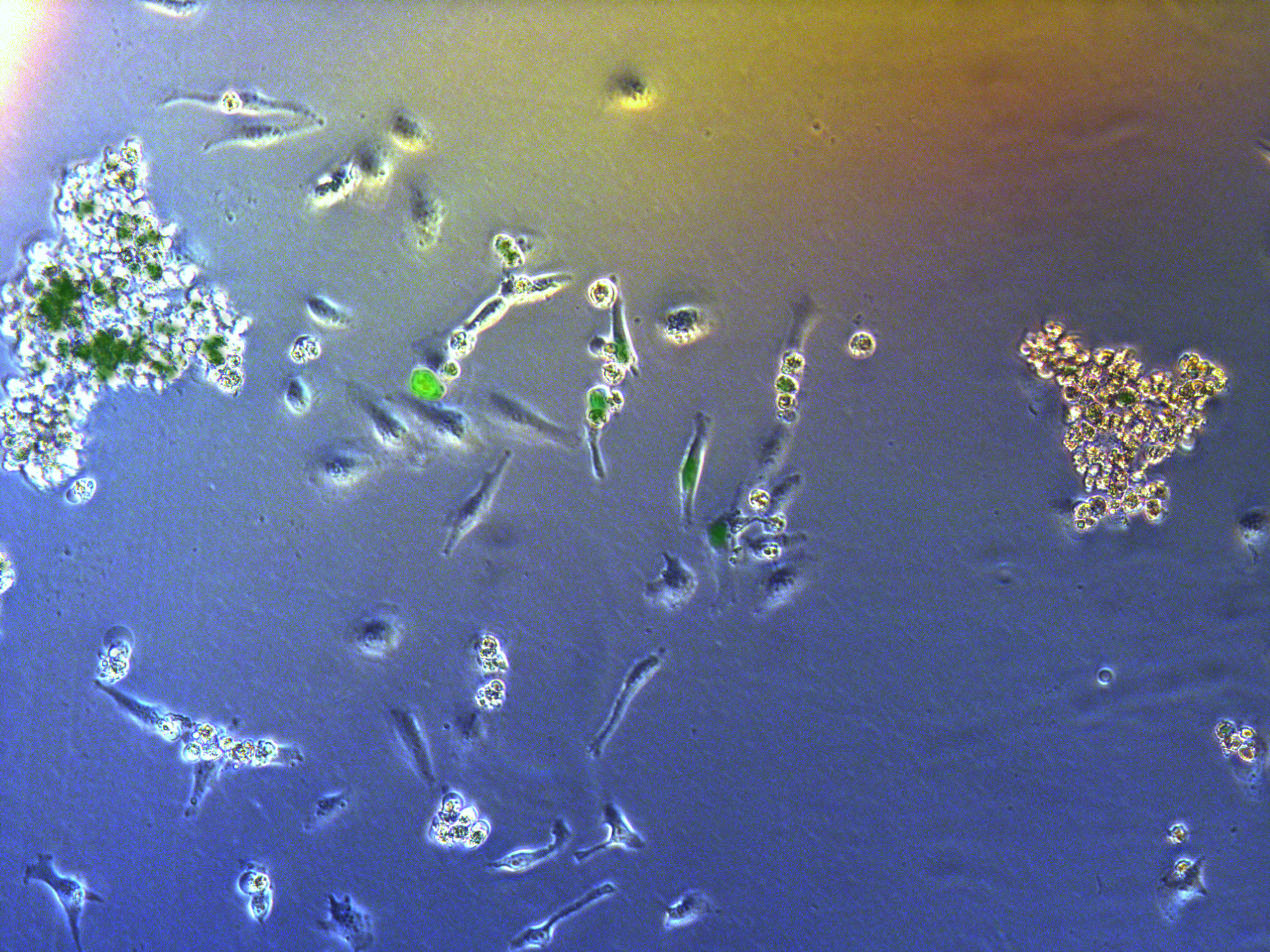
Figure . G2 pool cells on petri dish.

Figure . G3 pool cells on petri dish.

Figure . G4 pool cells on petri dish.
A considerable number of cells have been killed by antibiotcs. Those concentrated round cells in pictures are dead cells flouting in medium.
Monoclonal cells

Figure . Plate G1 seeded 50000 cells.
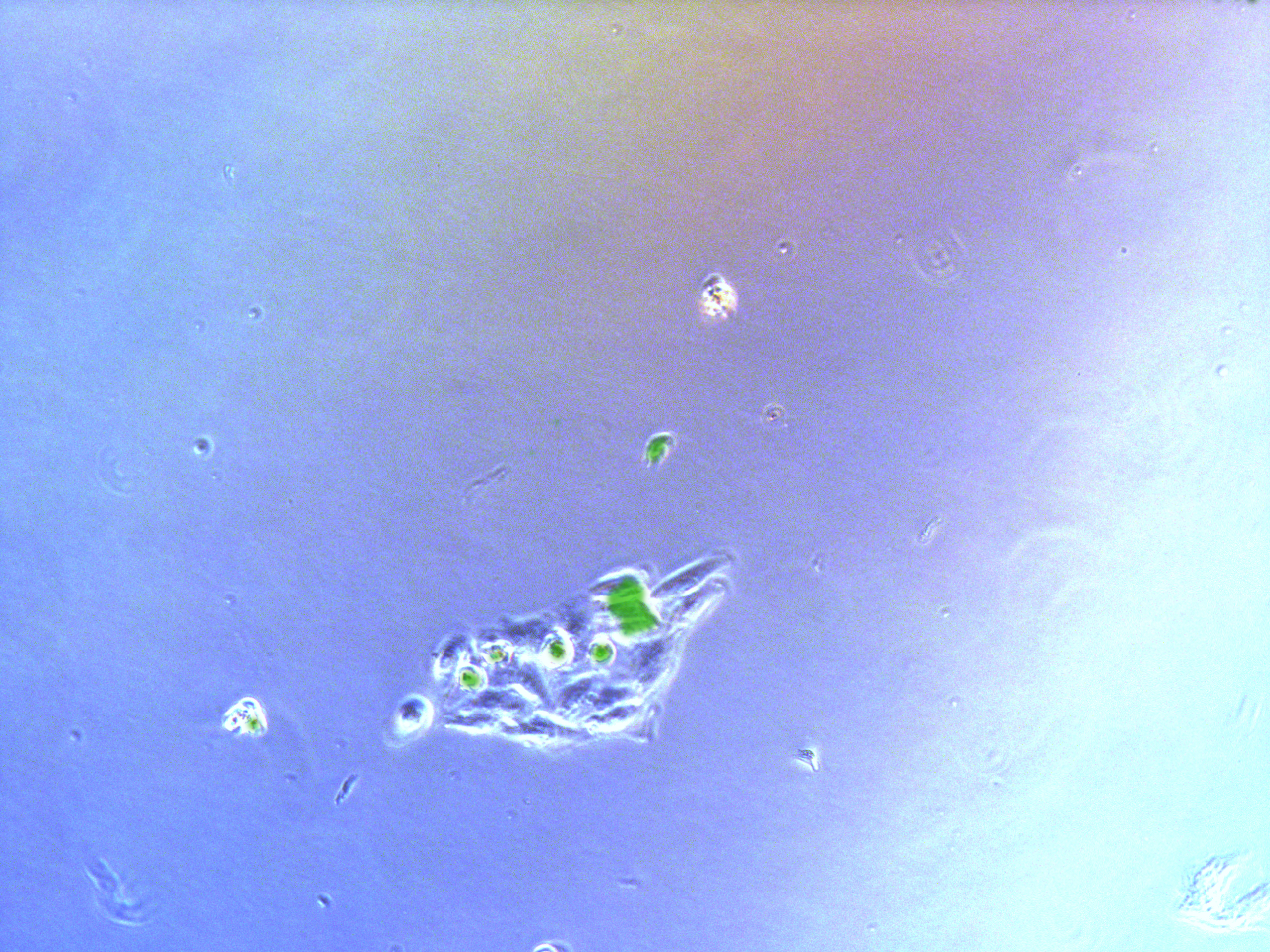
Figure . Plate G2 seeded 50000 cells.

Figure . Plate G3 seeded 50000 cells.
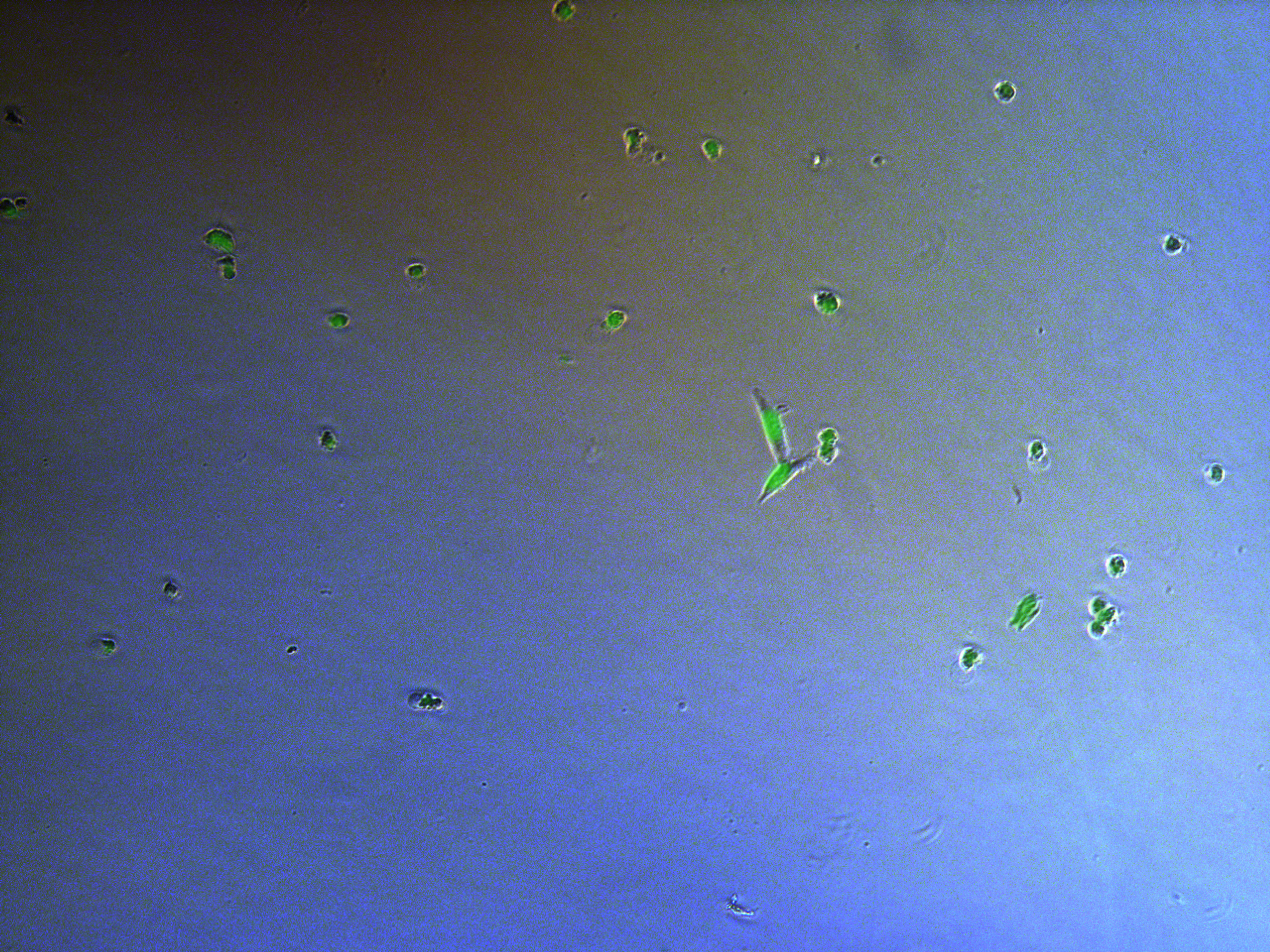
Figure . Plate G4 seeded 5000 cells.

Figure . Plate G4 seeded 50000 cells.
Cells on these plate has already grows into small monoclones. And some of clones having no fluorescence as well as resistance have not been killed by antibiotics. Clones and clones separate widely normally 2-3 clones per view.
Result
Plates to construct has a much higher concentration, esay to find a view with many cells. Group 1,3 has more cells with fluorescencs and more intense than group 2,4.
//TODO{
Fluorescent Observation
[2014 Aug 21st 12:00am]
The sixth day after transfection The third day selection
- G1-4 50,000 plate has many cells dead. Morethan half cells have been killed.
- Pool plate
- G5: Most cells are dead, only 1~2 clones per view field at 4x objective lens.
- G4: Many cells are dead, less than one clone every view field of 4x objective lens 10~20 cells per clone.
- G3: Many cells are dead and suspend in medium. 5~10 clone per view field. 30 cells per clone.
- G2: More cells than G4 but less than G3.
- G1: More cells than G3.
}//TODO
Change medium
[2014 Aug 21st 14:00pm]
All plate have been changed medium. The medium is seriously vaporated, only 3ml medium left in plate, 5ml orginally.
Seed plate for second transfection
[2014 Aug 22th 18:30pm]
The seventh day after transfection The forth day selection
Prepare for the second transfection.
We think that the first transfection may not be good enough, as a result, we prepare for the second time transfection. Just the same as the first time, we schemed to transfect HeLa cells with 3 different combination of plasmid.
| Group A | EGFP:Piggybac = 1:1 |
|---|---|
| Group B | Cas9:EGFP:Piggybac = 1:1:1 |
| Group C | Cas9:Piggybac = 1:1 |
We seed HeLa cells in 6-well-plate (6wells at total), 100,000 cells per well. For tansfection
- Procedure
- Wash cells with PBS, trypsin digestion, and medium to stop digestion.
- Discard the supernatant and add 8ml complete medium to resuspend the cells.
- Add 1ml PBS to every gap between well
- 1:10 dilution count the cell concentration with blood counting slide. Get the concentration: 760,000 cells/ml. 0.78ml cell suspendion + about 9ml DMEM with 10% FBS. Mix well and add 1.5ml per well, to mix well pepite the well gently.
Result
Cells seeded really even.
//TODO{
Fluorescence Observation
[2014 Aug 23rd 10:29] The eighth day after transfection The fifth day selection
After 5 days selection, almost every cell surviving expressing green fluorescence. Only a few cells on 10cm petri dish for monoclones do not express green fluorescence.
When peak up monoclones to construct a stable cell-line, we will check its green fluorescence under fluorescence microscope.
Monoclones
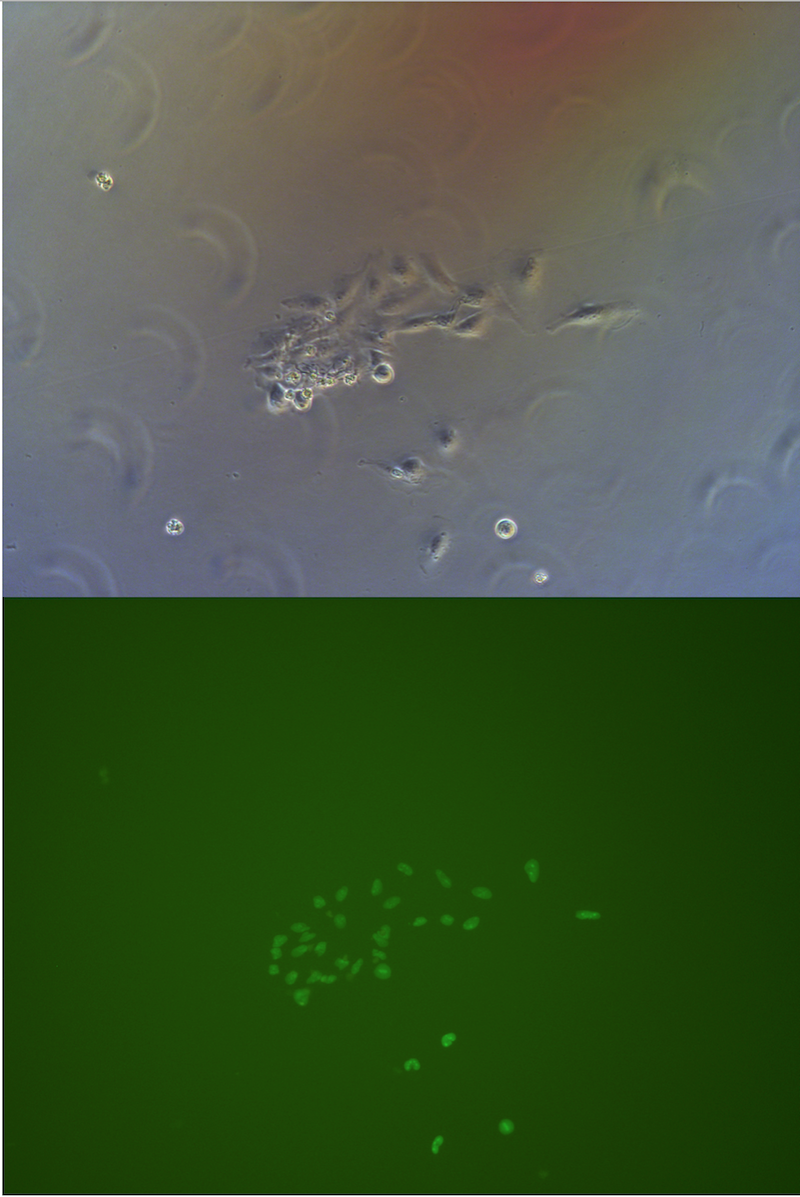
Figure . Picture of G1 monoclones, almost every cells has fluorescece.
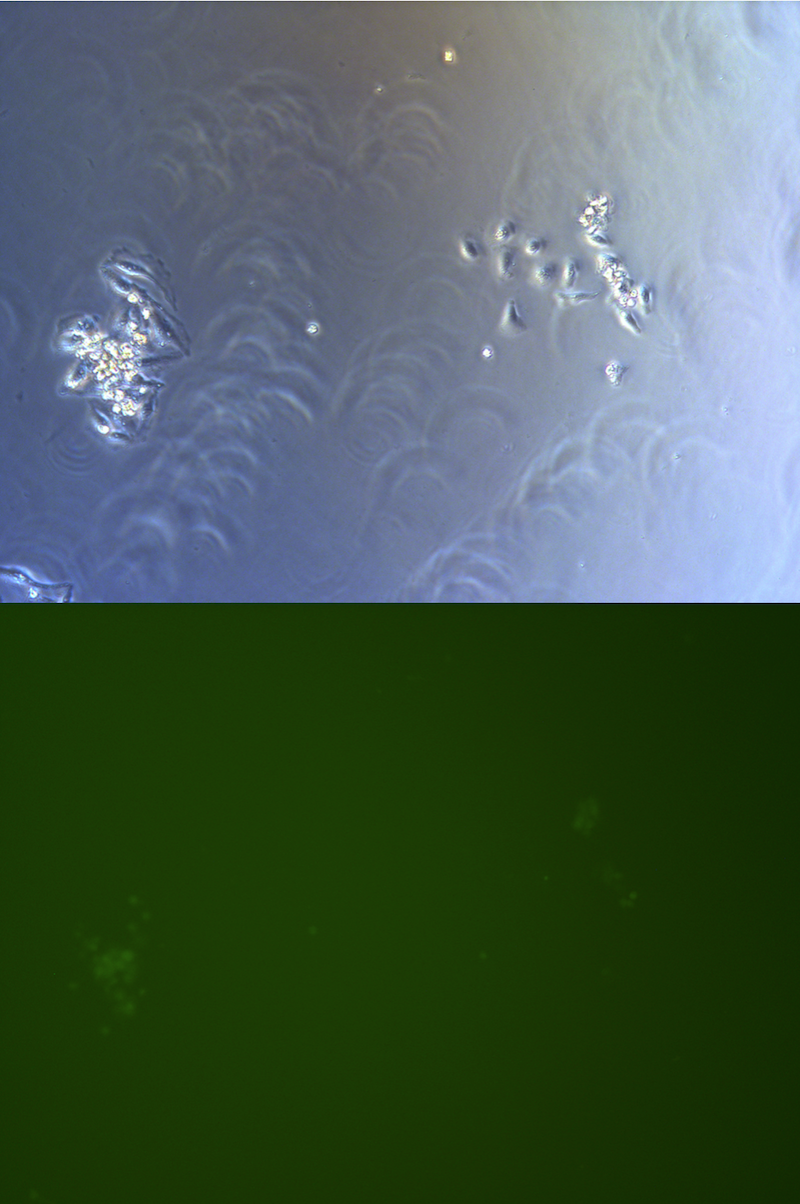
Figure . Picture of G2 monoclones, hardly to find cells expressing GFP.

Figure . Picture of G3 monoclones, almost every cells has GFP.

Figure . Picture of G4 monoclones, some of cells expressing GFP.
We observed and note clones expressing clones in all clones we observed under fluorescent microscope.
| Groups | With GFP | Without GFP |
|---|---|---|
| Group 1 | 11 | 12 |
| Group 2 | 4 | 11 |
| Group 3 | 10 | 10 |
| Group 4 | 9 | 11 |
Pool cells
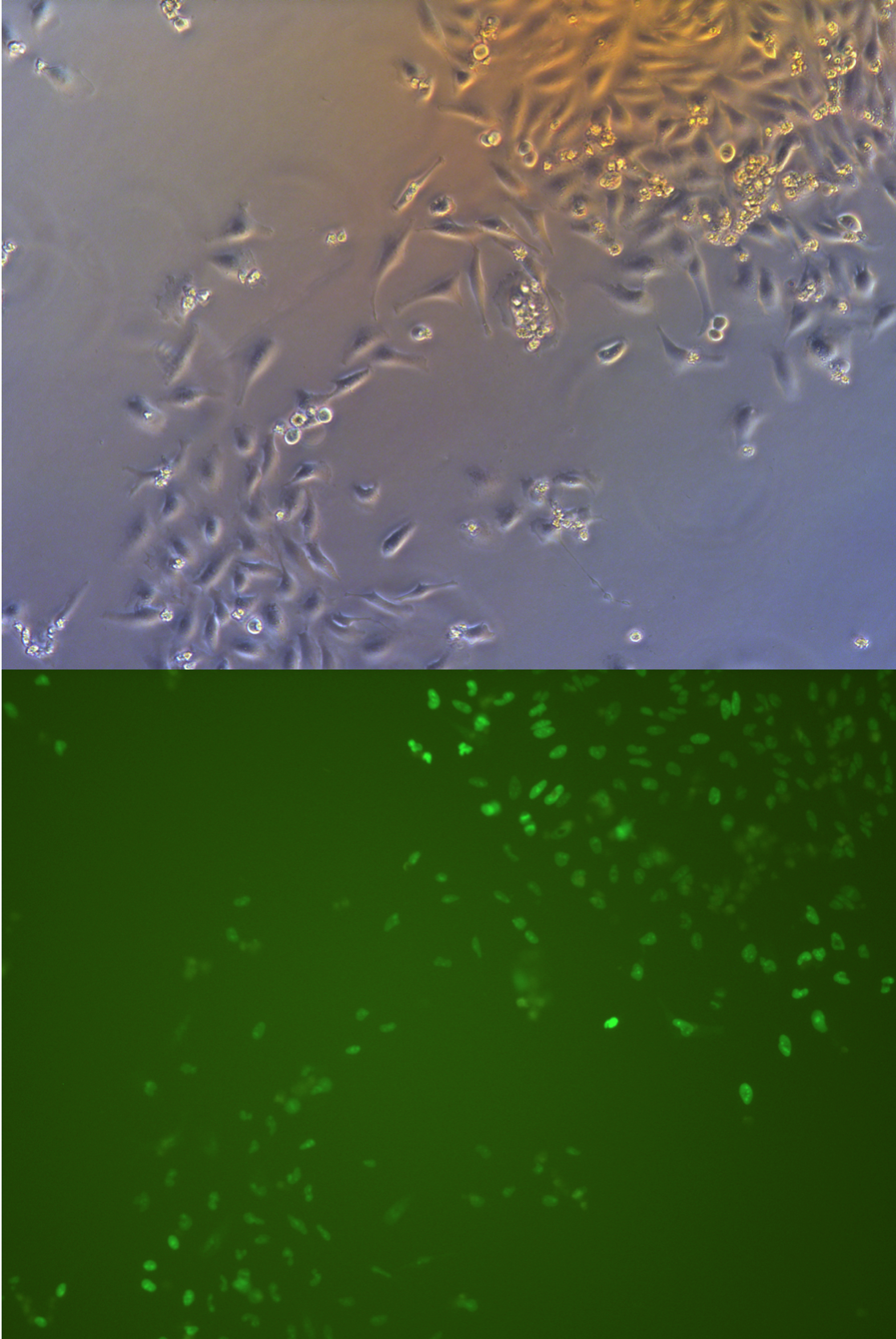
Figure .
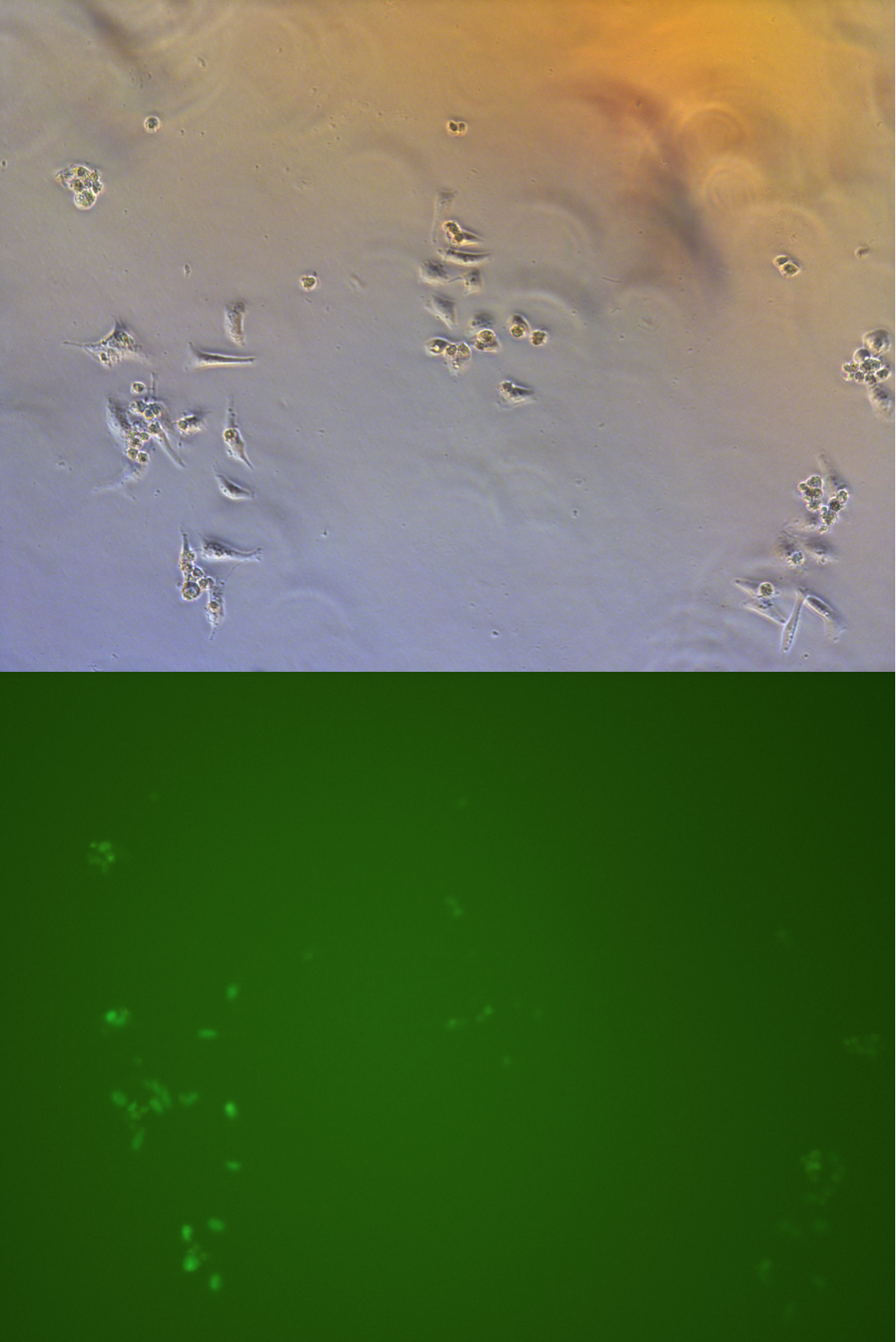
Figure .
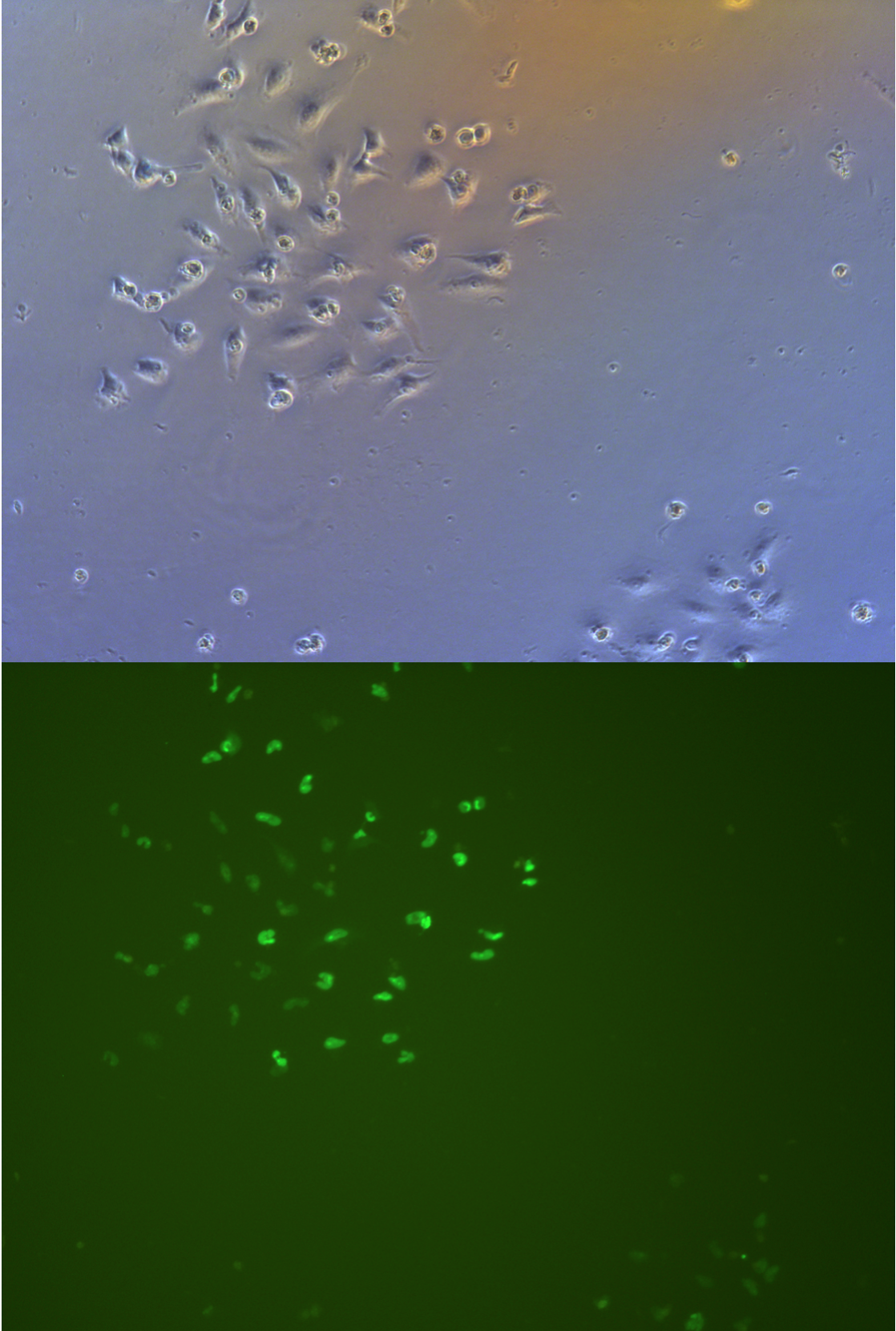
Figure .
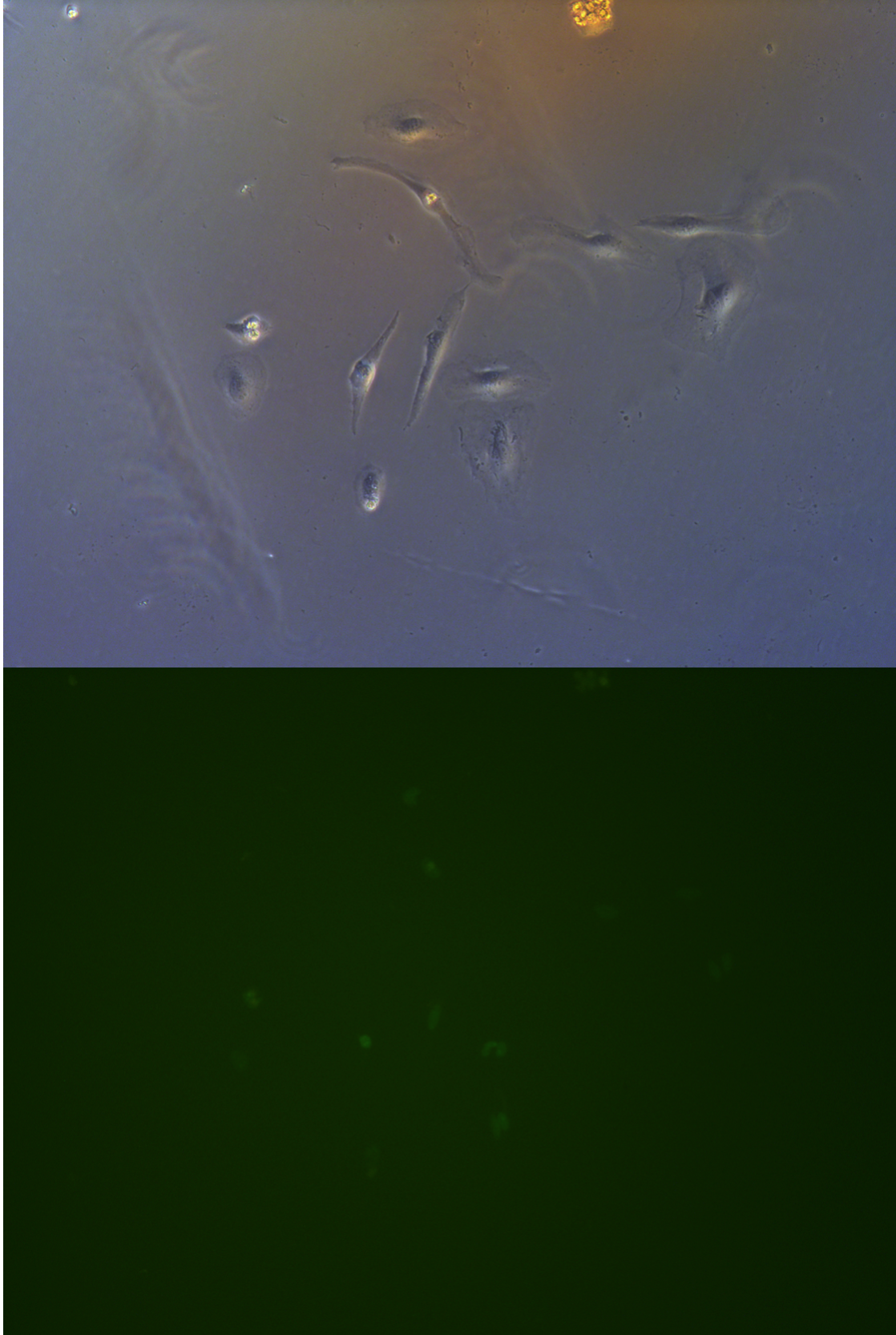
Figure .
//TODO}
Second transfection
[2014 Aug 25th 11:00am]
 "
"