Team:RHIT/Sustainability
From 2014.igem.org
| (50 intermediate revisions not shown) | |||
| Line 7: | Line 7: | ||
</style> | </style> | ||
| + | <style> | ||
| + | html { | ||
| + | background-color:#DFFFE9; | ||
| + | } | ||
| + | |||
| + | #content { | ||
| + | background-color:#DFFFE9; | ||
| + | } | ||
| + | |||
| + | #bodyContent { | ||
| + | background-color:#DFFFE9; | ||
| + | } | ||
| + | </style> | ||
<style type="text/css"> | <style type="text/css"> | ||
| Line 13: | Line 26: | ||
margin:0; | margin:0; | ||
width: 100%; | width: 100%; | ||
| - | background:# | + | background:#DFFFE9; |
} | } | ||
| Line 33: | Line 46: | ||
.accordion label { | .accordion label { | ||
display:block; | display:block; | ||
| - | font-size: | + | font-size:14px; |
| - | line-height: | + | line-height:14px; |
| - | background:# | + | background:#DFFFE9; |
text-shadow:1px 1px 1px rgba(255,255,255,0.3); | text-shadow:1px 1px 1px rgba(255,255,255,0.3); | ||
font-weight:700; | font-weight:700; | ||
| Line 45: | Line 58: | ||
.accordion ul li label:hover, .accordion [type=radio]:checked ~label, .accordion [type=checkbox]:checked ~ label { | .accordion ul li label:hover, .accordion [type=radio]:checked ~label, .accordion [type=checkbox]:checked ~ label { | ||
| - | background:# | + | background:#2CE46C; |
color:#FFFFFF; | color:#FFFFFF; | ||
text-shadow: 1px 1px 1px rgba(0,0,0,0.5); | text-shadow: 1px 1px 1px rgba(0,0,0,0.5); | ||
| Line 59: | Line 72: | ||
.accordion p { | .accordion p { | ||
color:#000000; | color:#000000; | ||
| - | margin:0 | + | margin:0 25px 10px; |
| + | text-align:left; | ||
} | } | ||
| Line 120: | Line 134: | ||
| - | + | <body> | |
| - | + | <div style="margin:20px 150px 10px"> | |
<h3 style="font-size:36px; margin-top:20px">PLA Recycling</h3> | <h3 style="font-size:36px; margin-top:20px">PLA Recycling</h3> | ||
<br> | <br> | ||
| + | <img src="http://i1265.photobucket.com/albums/jj502/bauhand/Sustdiagram_zpsa48511f4.png" align="left" width="400px"/> | ||
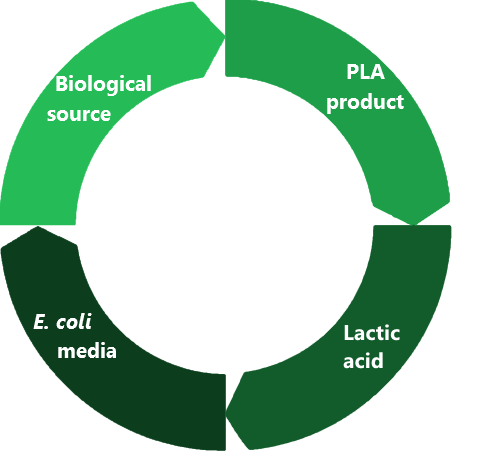
| + | <p>In the production of Victor the Vector, our team used a 3D printer to create parts for the device. This 3D printer used PLA (polylactic acid) plastic to print the parts.</p> | ||
| + | <br> | ||
| + | <p>PLA is a biodegradable polyester that is developed from various biological sources such as corn starch. PLA can be used for several plastic products such as cups, packaging material, and even as various parts of medical implants. It is a desirable replacement for several other plastics that may not be able to degrade.</p> | ||
| + | <br> | ||
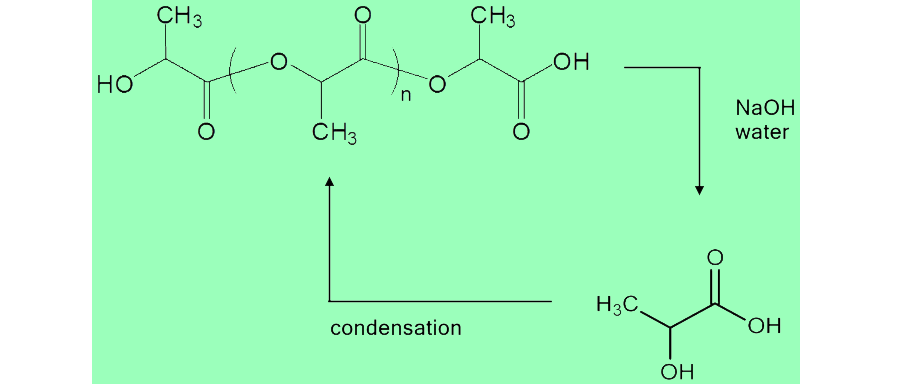
| + | <p>Due to the fact that PLA is produced from biological sources, it is reasonable that it could be broken down and recycled into the environment. Since PLA is a polymer of lactic acid, hydrolysis of the polymer will degrade it back into lactic acid. Our team decided to use the scrap pieces of PLA from the 3D printing, and convert it back into lactate, which could then be used in the media for our <i>E. coli</i> cells.</p> | ||
| + | <br> | ||
| + | <p>This was done by a simple hydrolysis reaction, adding base to the polymer to break it down, then neutralizing the solution with acid and filtering. The reaction is shown below along with the protocols used to degrade and characterize the solution.</p> | ||
| + | <br clear="all"> | ||
| + | </div> | ||
| + | <div style="margin:10px 250px 100px 250px"> | ||
| + | <img src="http://i1265.photobucket.com/albums/jj502/bauhand/PLAreaction_zps5c13ea5d.png" width="650px" align="right"/> | ||
| - | + | <p style="font-size:20px"><b><u>Protocols</u></b></p> | |
| - | < | + | |
<div class="accordion vertical"> | <div class="accordion vertical"> | ||
<ul> | <ul> | ||
| Line 162: | Line 187: | ||
<div class="content"> | <div class="content"> | ||
<p><p>The filtered solution was measured in a polarimer. The optical rotation results are as follows.</p> | <p><p>The filtered solution was measured in a polarimer. The optical rotation results are as follows.</p> | ||
| - | <table | + | <table text-align="center"> |
<tr style="background-color:#D8D8D8; border:1px solid black"> | <tr style="background-color:#D8D8D8; border:1px solid black"> | ||
<th style="border:1px solid black">pH</th> | <th style="border:1px solid black">pH</th> | ||
| Line 191: | Line 216: | ||
<label for="checkbox-3">LDH Assay</label> | <label for="checkbox-3">LDH Assay</label> | ||
<div class="content"> | <div class="content"> | ||
| - | <p> | + | <p><p>Run a test assay using known concentrations of lactate.</p> |
| - | </p> | + | <p>Make up the following stock solutions:</p> |
| + | <p><u>Lactate Stock (120mM):</u></p> | ||
| + | <ul> | ||
| + | <li>.57606g lactate</li> | ||
| + | <li>50 mL 10mM Tris-HCl (pH 8.6)</li> | ||
| + | </ul> | ||
| + | <p><u>NAD+ Stock (12mM):</u></p> | ||
| + | <ul> | ||
| + | <li>.0398g NAD+</li> | ||
| + | <li>5 mL 10mM Tris-HCl (pH 8.6)</li> | ||
| + | </ul> | ||
| + | <p><u>Bicarbonate Stock (18mM):</u> | ||
| + | <ul> | ||
| + | <li>.00302g NaHCO<sub>3</sub></li> | ||
| + | <li>2 mL 10mM Tris-HCl (pH 8.6)</li> | ||
| + | </ul> | ||
| + | <p>Using the lactate stock solution, make up the following dilutions: 1mM, .5mM, .2mM, .1mM, 0mM</p> | ||
| + | <p>In a cuvette, add 0.6 mL 1mM dilution, 0.4 mL NAD+ stock, and 0.2 mL Bicarbonate stock. Place the cuvette in the spectrophotometer and measure the absorbance. Leaving the cuvette in the spectrophotometer, add 10 µL lactate dehydrogenase (LDH) enzyme. Allow the reaction to run in the cuvette until the absorbance plateaus. Repeat procedure with remaining dilutions in separate cuvettes.</p> | ||
| + | <p>The following is the data from the test run:</p> | ||
| + | <table> | ||
| + | <tr style="background-color:#DFFFE9; border:1px solid black"> | ||
| + | <th style="border:1px solid black">Concentrations</th> | ||
| + | <th style="border:1px solid black">OD Before LDH</th> | ||
| + | <th style="border:1px solid black">OD After LDH</th> | ||
| + | <th style="border:1px solid black">Difference</th> | ||
| + | </tr> | ||
| + | |||
| + | <tr style="background-color:#DFFFE9; border:1px solid black"> | ||
| + | <td style="border:1px solid black">0 mM</td> | ||
| + | <td style="border:1px solid black">0.242</td> | ||
| + | <td style="border:1px solid black">0.242</td> | ||
| + | <td style="border:1px solid black">0</td> | ||
| + | </tr> | ||
| + | |||
| + | <tr style="background-color:#DFFFE9; border:1px solid black"> | ||
| + | <td style="border:1px solid black">.1 mM</td> | ||
| + | <td style="border:1px solid black">0.284</td> | ||
| + | <td style="border:1px solid black">0.367</td> | ||
| + | <td style="border:1px solid black">0.083</td> | ||
| + | </tr> | ||
| + | |||
| + | <tr style="background-color:#DFFFE9; border:1px solid black"> | ||
| + | <td style="border:1px solid black">.2 mM</td> | ||
| + | <td style="border:1px solid black">0.299</td> | ||
| + | <td style="border:1px solid black">0.401</td> | ||
| + | <td style="border:1px solid black">0.102</td> | ||
| + | </tr> | ||
| + | |||
| + | <tr style="background-color:#DFFFE9; border:1px solid black"> | ||
| + | <td style="border:1px solid black">.5 mM</td> | ||
| + | <td style="border:1px solid black">0.31</td> | ||
| + | <td style="border:1px solid black">0.454</td> | ||
| + | <td style="border:1px solid black">0.144</td> | ||
| + | </tr> | ||
| + | |||
| + | <tr style="background-color:#DFFFE9; border:1px solid black"> | ||
| + | <td style="border:1px solid black">1 mM</td> | ||
| + | <td style="border:1px solid black">0.244</td> | ||
| + | <td style="border:1px solid black">0.483</td> | ||
| + | <td style="border:1px solid black">0.239</td> | ||
| + | </tr> | ||
| + | |||
| + | <tr style="background-color:#DFFFE9; border:1px"> | ||
| + | <td style="border:1px solid black">1 mM</td> | ||
| + | <td style="border:1px solid black">0.297</td> | ||
| + | <td style="border:1px solid black">0.54</td> | ||
| + | <td style="border:1px solid black">0.243</td> | ||
| + | </tr> | ||
| + | </table> | ||
| + | |||
| + | |||
| + | </p><br> | ||
| + | <p>The same protocol was replicated using the lactic acid solution obtained from PLA degradation and purification. Dilutions were made until the absorbance spectrum showed a useful peak at 340 nm. This dilution was then diluted by a factor of two to obtain further data. The assay will be run again to collect more data points before analysis. | ||
</div> | </div> | ||
</li> | </li> | ||
</ul> | </ul> | ||
</div> | </div> | ||
| - | + | </div> | |
</body> | </body> | ||
</html> | </html> | ||
Latest revision as of 02:10, 17 October 2014
 "
"