In the production of Victor the Vector, our team used a 3D printer to create parts for the device. This 3D printer used PLA (polylactic acid) plastic to print the parts.
PLA is a biodegradable polyester that is developed from various biological sources such as corn starch. PLA can be used for several plastic products such as cups, packaging material, and even as various parts of medical implants. It is a desirable replacement for several other plastics that may not be able to degrade.
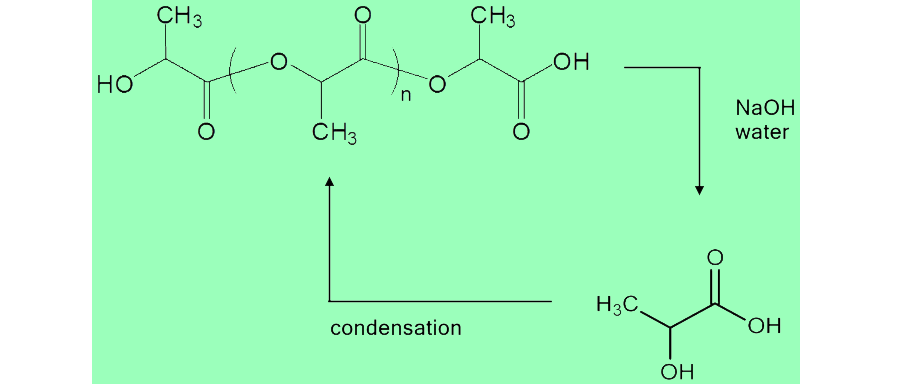
Due to the fact that PLA is produced from biological sources, it is reasonable that it could be broken down and recycled into the environment. Since PLA is a polymer of lactic acid, hydrolysis of the polymer will degrade it back into lactic acid. Our team decided to use the scrap pieces of PLA from the 3D printing, and convert it back into lactate, which could then be used in the media for our E. coli cells.
This was done by a simple hydrolysis reaction, adding base to the polymer to break it down, then neutralizing the solution with acid and filtering. The reaction is shown below along with the protocols used to degrade and characterize the solution.
Protocols
To a 1 L Erlenmeyer flask add:
Stir reaction, heating to 50-60°C, overnight or until all plastic has visibly dissolved.
Neutralize with HCl to a pH of 5-6.
Vacuum filter solution 2-3 times, dispose of any solid collected.
Using a sterile syringe, filter solution into a jar to store until needed.
The filtered solution was measured in a polarimer. The optical rotation results are as follows.
| pH | Optical Rotation |
|---|---|
| 2.5 | .12 |
| 3.5 | -.13 |
| 4.21 | -.26 |
This suggests that the solution did in fact contain lactate because a pH change made a significant difference in the optical rotation of the solution.
Run a test assay using known concentrations of lactate.
Make up the following stock solutions:
Lactate Stock (120mM):
NAD+ Stock (12mM):
Bicarbonate Stock (18mM):
Using the lactate stock solution, make up the following dilutions: 1mM, .5mM, .2mM, .1mM, 0mM
In a cuvette, add 0.6 mL 1mM dilution, 0.4 mL NAD+ stock, and 0.2 mL Bicarbonate stock. Place the cuvette in the spectrophotometer and measure the absorbance. Leaving the cuvette in the spectrophotometer, add 10 µL lactate dehydrogenase (LDH) enzyme. Allow the reaction to run in the cuvette until the absorbance plateaus. Repeat procedure with remaining dilutions in separate cuvettes.
The following is the data from the test run:
| Concentrations | OD Before LDH | OD After LDH | Difference |
|---|---|---|---|
| 0 mM | 0.242 | 0.242 | 0 |
| .1 mM | 0.284 | 0.367 | 0.083 |
| .2 mM | 0.299 | 0.401 | 0.102 |
| .5 mM | 0.31 | 0.454 | 0.144 |
| 1 mM | 0.244 | 0.483 | 0.239 |
| 1 mM | 0.297 | 0.54 | 0.243 |
The same protocol was replicated using the lactic acid solution obtained from PLA degradation and purification. Dilutions were made until the absorbance spectrum showed a useful peak at 340 nm. This dilution was then diluted by a factor of two to obtain further data. The assay will be run again to collect more data points before analysis.