|
|
| (21 intermediate revisions not shown) |
| Line 32: |
Line 32: |
| | The ''upp'' gene from Bacillus subtilis W168 encodes for a Uracilphosphoribosyl transferase (UPRTase). | | The ''upp'' gene from Bacillus subtilis W168 encodes for a Uracilphosphoribosyl transferase (UPRTase). |
| | Its key reaction in uracil salvage is the reaction of a uracil molecule with a 5'-phosphoribosyl-a-1- pyrophosphate (PRPP) molecule, resulting in the formation of UMP. | | Its key reaction in uracil salvage is the reaction of a uracil molecule with a 5'-phosphoribosyl-a-1- pyrophosphate (PRPP) molecule, resulting in the formation of UMP. |
| - | A second locus, the ''pyrR'' gene, encoding a second UPRTase has been identified. However it has been shown, that the UPRTase derived from ''pyrR'' locus has an influence on overall UPRTase activity of < 1 %. (J Martinussen, P Glaser, P S Andersen and H H Saxild J. Bacteriol. 1995, 177(1):271.) | + | A second locus, the ''pyrR'' gene, encoding a second UPRTase has been identified. However it has been shown, that the UPRTase derived from ''pyrR'' locus has an influence on overall UPRTase activity of < 1 %. [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC176586/] |
| | This makes the ''upp''-derived UPRTase the only physiologically relevant catalyst for UPRTase activity. | | This makes the ''upp''-derived UPRTase the only physiologically relevant catalyst for UPRTase activity. |
| | Exposure to the pyrimidine analogue 5-Fluorouracil UPRTase results in production of 5-fluoro-dUMP, a very potent inhibitor of the thymidylate synthase (Neuhard, J. (1983) Utilization of preformed pyrimidine bases and nucleosides. In Metabolism of Nucleotides, Nucleosides and Nucleobases in Microorganisms. Munch- Petersen, A. (eds). New York: Academic Press, pp. 95– 148. ). As a result 5-FU is toxic to the ''Bacillus subtilis'' W168 strain. | | Exposure to the pyrimidine analogue 5-Fluorouracil UPRTase results in production of 5-fluoro-dUMP, a very potent inhibitor of the thymidylate synthase (Neuhard, J. (1983) Utilization of preformed pyrimidine bases and nucleosides. In Metabolism of Nucleotides, Nucleosides and Nucleobases in Microorganisms. Munch- Petersen, A. (eds). New York: Academic Press, pp. 95– 148. ). As a result 5-FU is toxic to the ''Bacillus subtilis'' W168 strain. |
| - | ''B. subtilis'' 5FU-resistant (5FUR) mutants selected on low drug concentration (10 mM 5FU) are UPRTase-defective. (Nygaard, P. (1993) Purine and pyrimidine salvage pathways. In Bacillus Subtilis and Other Gram-Positive Bacteria: Biochemistry, Physiology, and Molecular Genetics. Sonenshein, A.L., Hoch, J.A. and Losick, R., (eds). Washington, DC: American Society for Microbiology, pp. 359–378. ) | + | ''B. subtilis'' 5FU-resistant (5FUR) mutants selected on low drug concentration (10 mM 5FU) are UPRTase-defective. [http://www.nature.com/nature/journal/v390/n6657/abs/390249a0.html]. |
| - | This has made ''upp'' a go to choice for negative selection in combination with an ''B. subtilis'' W168 ''Δupp'' strain. So far it has been used to make clean in-frame deletions and point mutations. (A new mutation delivery system for genome-scale approaches in Bacillus subtilis Céline Fabret,† S. Dusko Ehrlich and Philippe Noirot* Génétique Microbienne, INRA, Domaine de Vilvert, 78352 Jouy en Josas Cedex, France. ) | + | This has made ''upp'' a go to choice for negative selection in combination with an ''B. subtilis'' W168 ''Δupp'' strain. So far it has been used to make clean in-frame deletions and point mutations. [http://www.ncbi.nlm.nih.gov/pubmed/12366828] |
| | | | |
| | However, to our knowledge, no application using ''upp'' for clean insertions has been established so far. | | However, to our knowledge, no application using ''upp'' for clean insertions has been established so far. |
| | | | |
| - | The I-SceI Restricition endonuclease has a highly specific recognition sequence of 18 nucleotides. No such sequence is present in the ''B.subtilis'' W168 strain. It creates a double strand break at targeted location, which leads to an increased rate of repair at the specific site. By this, the rate of homologous recombination is increased by a factor of 100. (Establishment of a Markerless Mutation Delivery System in Bacillus subtilis Stimulated by a Double-Strand Break in the Chromosome Ting Shi1,2,3,4., Guanglu Wang1,2,3,4., Zhiwen Wang1,2,3,4*, Jing Fu1,2,3,4, Tao Chen1,2,3,4, Xueming Zhao1,2,3,4 ) | + | The I-SceI Restricition endonuclease has a highly specific recognition sequence of 18 nucleotides. No such sequence is present in the ''B.subtilis'' W168 strain. It creates a double strand break at targeted location, which leads to an increased rate of repair at the specific site. By this, the rate of homologous recombination is increased by a factor of 100. [http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0081370] |
| | <html> | | <html> |
| | </article> | | </article> |
| Line 52: |
Line 52: |
| | Integration of those basic vectors is achieved via homologous recombination between the ''amyE''/''lacA''/''thrC'' locus, respectively, and the corresponding up and down fragments on the vector. This leads to an insertion of the gene of interest within the RCF10 compatible multiple cloning site and the resistance for positive selection (Cm<sup>r</sup>/MLS<sup>r</sup>/Spec<sup>r</sup>). | | Integration of those basic vectors is achieved via homologous recombination between the ''amyE''/''lacA''/''thrC'' locus, respectively, and the corresponding up and down fragments on the vector. This leads to an insertion of the gene of interest within the RCF10 compatible multiple cloning site and the resistance for positive selection (Cm<sup>r</sup>/MLS<sup>r</sup>/Spec<sup>r</sup>). |
| | | | |
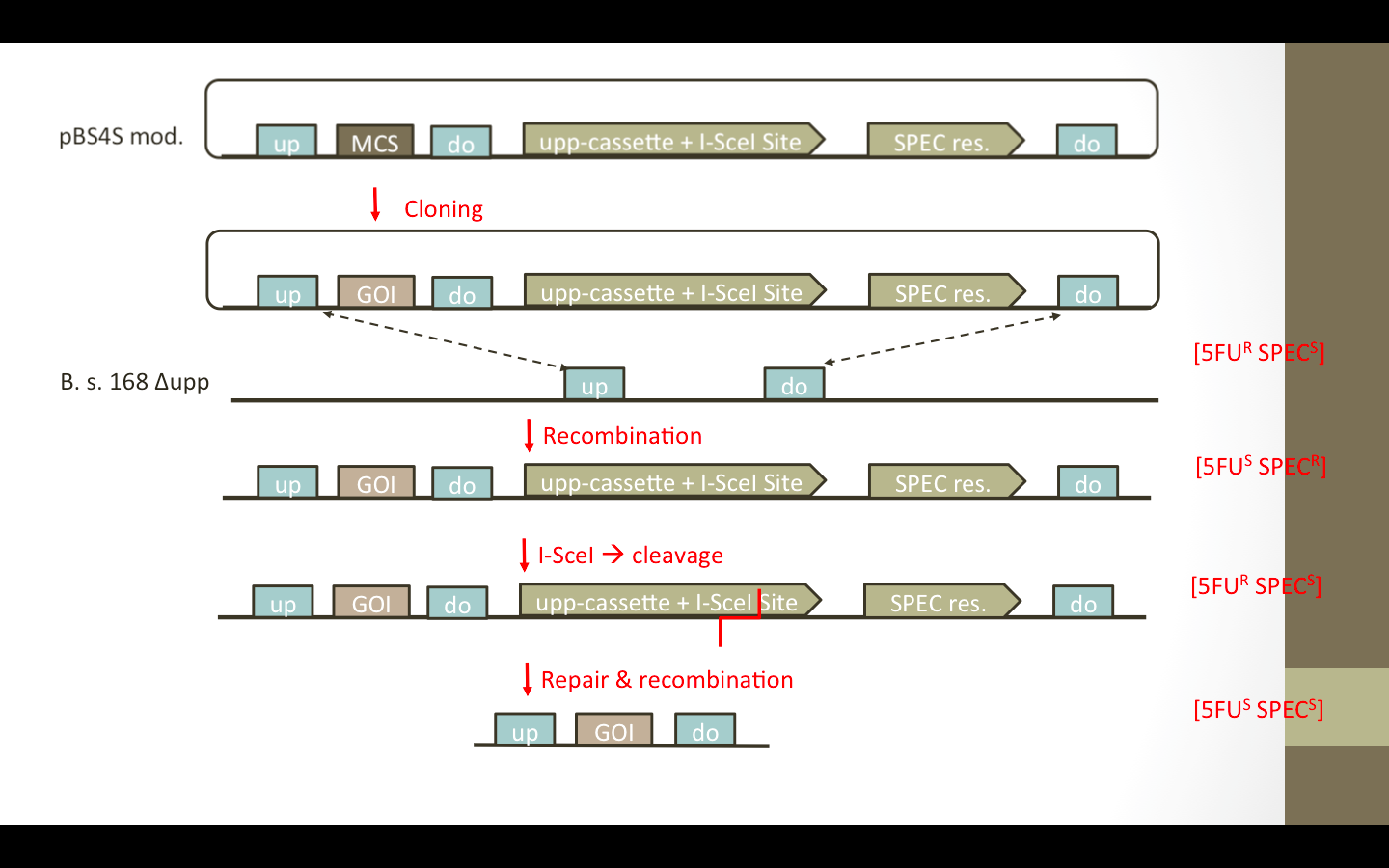
| - | We wanted to add the ''upp''-cassette, containing an I-SceI site, as well as an additional up/down fragment to the vector. The desired vectors are presented in Fig. 1. The ''upp''-cassette will allow negative selection in 5FU media. The contained I-SceI site will be cut by the I-SceI restriction endonuclease which is encoded on a helper plasmid pEBS-cop1. (Establishment of a Markerless Mutation Delivery System in Bacillus subtilis Stimulated by a Double-Strand Break in the Chromosome Ting Shi1,2,3,4., Guanglu Wang1,2,3,4., Zhiwen Wang1,2,3,4*, Jing Fu1,2,3,4, Tao Chen1,2,3,4, Xueming Zhao1,2,3,4) (Figure) | + | We wanted to add the ''upp''-cassette, containing an I-SceI site, as well as an additional up/down fragment to the vector. The desired vectors are presented in Fig. 1. The ''upp''-cassette will allow negative selection in 5FU media. The contained I-SceI site will be cut by the I-SceI restriction endonuclease which is encoded on a helper plasmid pEBS-cop1. [http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0081370] (Figure) |
| | | | |
| | | | |
| | | | |
| - | [[File:LMU14 RFCloning pEBS-cop1.jpg|thumb|center|pEBS-cop1. Plasmid containing ...]] | + | [[File:LMU14 RFCloning pEBS-cop1.jpg|thumb|center|pEBS-cop1. Plasmid containing the ''i-sceI'' gene]] |
| | Cloning Strategy | | Cloning Strategy |
| | | | |
| Line 104: |
Line 104: |
| | </html> | | </html> |
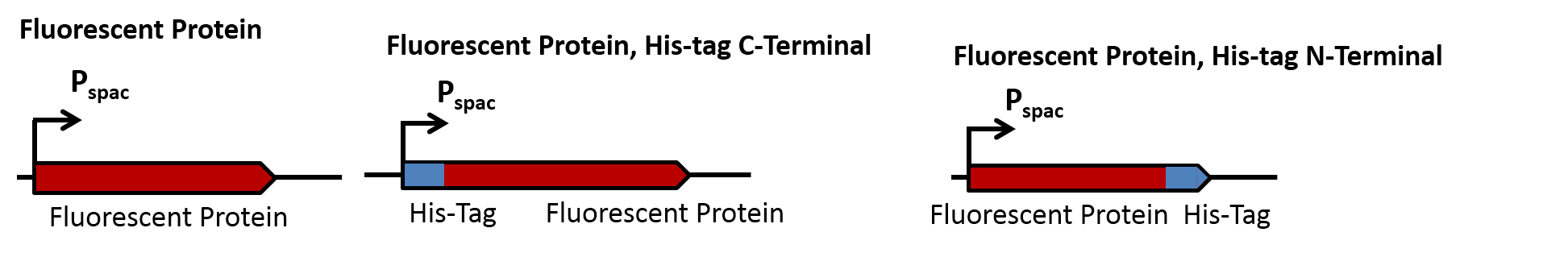
| | Seven different FPs (Table 1) where chosen and either obtained from the registry or from the lab group Mascher. | | Seven different FPs (Table 1) where chosen and either obtained from the registry or from the lab group Mascher. |
| - | The BioBrick [http://parts.igem.org/Part:BBa_E1010 BBa_E1010] was mutated via site directed mutagenesis by overlap extension PCR in order to delete two AgeI-Restriction sites and make the BioBrick compatible for the Freiburg standard RFC25. This improved BioBrick is called [http://parts.igem.org/Part:BBa_K1351021 BBa_K1351021] | + | The BioBrick <partinfo>BBa_E1010</partinfo>was mutated via site directed mutagenesis by overlap extension PCR in order to delete two AgeI-Restriction sites and make the BioBrick compatible for the Freiburg standard RFC25. This improved BioBrick is called <html><a href="http://parts.igem.org/Part:BBa_K1351021">BBa_K1351021</a></html> |
| - | | + | The BioBricks and [http://parts.igem.org/Part:BBa_K592100 BBa_K592100] (mTagBFP) where both provided with the necessary overhangs for the Freiburg standard, the other FPs where already provided with the proper restriction overhangs. |
| - | The BioBricks [http://parts.igem.org/Part:BBa_E1010 BBa_E1010] <html> (DsRed) </html> and [http://parts.igem.org/Part:BBa_K592100 BBa_K592100] (mTagBFP) where both provided with the necessary overhangs for the Freiburg standard, the other FPs where already provided with the proper restriction overhangs.
| + | |
| | These seven FPs where combined C- and N-terminally with a His-Tag ([http://parts.igem.org/wiki/index.php?title=Part:BBa_K823037 BBa_K823037]) and cloned into the vector pBS0K-Pspac ([http://parts.igem.org/wiki/index.php?title=Part:BBa_K1351040 BBa_K1351040]) and transformed into ''B. subtilis''. | | These seven FPs where combined C- and N-terminally with a His-Tag ([http://parts.igem.org/wiki/index.php?title=Part:BBa_K823037 BBa_K823037]) and cloned into the vector pBS0K-Pspac ([http://parts.igem.org/wiki/index.php?title=Part:BBa_K1351040 BBa_K1351040]) and transformed into ''B. subtilis''. |
| | | | |
| Line 165: |
Line 164: |
| | | | |
| | | | |
| - | [1]
| + | |
| | | | |
| | [[File:LMU14_FP_Gesamtkonstrukt.png|center|800px]] | | [[File:LMU14_FP_Gesamtkonstrukt.png|center|800px]] |
| Line 190: |
Line 189: |
| | | | |
| | This problem will be solved by recloning the FPs into the vector pBS1C ([http://parts.igem.org/wiki/index.php?title=Part:BBa_K823023 BBa_K823023]) together with the xylose-inducible promoter Pxyl([http://parts.igem.org/wiki/index.php?title=Part:BBa_K1351039 BBa_K1351039]). | | This problem will be solved by recloning the FPs into the vector pBS1C ([http://parts.igem.org/wiki/index.php?title=Part:BBa_K823023 BBa_K823023]) together with the xylose-inducible promoter Pxyl([http://parts.igem.org/wiki/index.php?title=Part:BBa_K1351039 BBa_K1351039]). |
| | + | <html> |
| | + | </article> |
| | + | </div> |
| | + | </section> |
| | + | </div> |
| | + | </section> |
| | + | <section class="bg-color-2"> |
| | + | <div> |
| | + | </html> |
| | + | |
| | + | == Linkers == |
| | + | Linkers are short peptides used to fuse two protein domains (or even whole proteins). They are designed not to interact with the fused parts. A linker of the wrong length can lead to sterically inactivated (if the linker is too short) or instable (if it is too long) constructs. Last but not least, linkers need to exhibit a certain flexibility. We evaluated a set of linkers partially already found in the registry using FRET. |
| | + | <html> |
| | + | <section class="accordion"> |
| | + | <div> |
| | + | <input id="linker-bground" name="linker-accord" type="checkbox" /> |
| | + | <label for="linker-bground">Background</label> |
| | + | <article class="ac-small"> |
| | + | </html> |
| | + | Förster Resonance Energy Transfer (FRET) is a phenomenon that occurs when two fluorophores with overlapping spectra are not more than 10 nanometers apart. When the donor fluorophore gets excited, and FRET happens, it does not emit all the energy as light, it transferres a part of the energy non-radiatively to the acceptor fluorophore, which starts fluorescing. The efficiency of this energy transfer decays with the sixth power of the distance between donor and acceptor. This makes FRET an ideal tool to measure or verify very short distances, like the ones between interacting proteins ''in vivo''. A common application would be tagging two supposedely interacting proteins with the FRET couple and measuring acceptor fluorescence. Obviously this technique lends itself well to the evaluation of linkers. |
| | + | <html> |
| | + | </article> |
| | + | </div> |
| | + | <div> |
| | + | <input id="linker-design" name="linker-accord" type="checkbox" /> |
| | + | <label for="linker-design">Design</label> |
| | + | <article class="ac-small"> |
| | + | </html> |
| | + | |
| | + | mTurquoise (donor) and mNeonGreen (acceptor) were used as FRET-couple for this study. Translational fusion constructs were created according to a modified RFC25 standard. From the registry we took the linkers [http://parts.igem.org/Part:BBa_K243004 BBa_K243004], [http://parts.igem.org/Part:BBa_K243005 BBa_K243005], [http://parts.igem.org/Part:BBa_K243006 BBa_K243006], [http://parts.igem.org/Part:BBa_K157009 BBa_K157009], [http://parts.igem.org/Part:BBa_K157013 BBa_K157013], [http://parts.igem.org/Part:BBa_K243029 BBa_K243029] and [http://parts.igem.org/Part:BBa_K243030 BBa_K243030]. We also used this method to characterize our own linkers [http://parts.igem.org/Part:BBa_K1351009 BBa_K1351009] and [http://parts.igem.org/Part:BBa_K1351035 BBa_K1351035]. For each linker a construct with mTurquise fused to it N-terminally and mNeonGreen C-terminally was created, as well as a construct where the FPs were oriented the other way around. |
| | + | <html> |
| | + | </article> |
| | + | </div> |
| | + | <div> |
| | + | <input id="linkers-results" name="linkers-accord" type="checkbox" /> |
| | + | <label for="linkers-results">Results</label> |
| | + | <article class="ac-small"> |
| | + | </html> |
| | + | The constructs have been created, and fluorescence has been observed. Due to time concerns, the FRET-assay has not yet been performed. |
| | <html> | | <html> |
| | </article> | | </article> |
| Line 211: |
Line 249: |
| | <article class="ac-small"> | | <article class="ac-small"> |
| | </html> | | </html> |
| - | RBS are short nucleotide sequences that are complemetary to the 3' end of 16S rRNA, and thus are bound by the ribosome during translation initiation. The RBS is usually found ~8 nucleotides upstream of the start codon in procaryotes. In ''B. subtilis'' the perfect consensus RBS is AAGGAGGGATA (Bba_K1351028). | + | RBS are short nucleotide sequences that are complemetary to the 3' end of 16S rRNA, and thus are bound by the ribosome during translation initiation. The RBS is usually found ~8 nucleotides upstream of the start codon in procaryotes. In ''B. subtilis'' the perfect consensus RBS is AAGGAGGGATA ([http://parts.igem.org/Part:BBa_K1351028 Bba_K1351028]). |
| | <html> | | <html> |
| | </article> | | </article> |
| Line 220: |
Line 258: |
| | <article class="ac-small"> | | <article class="ac-small"> |
| | </html> | | </html> |
| - | Using the Salis lab's [https://salis.psu.edu/software/ RBS calculator] we calculated our RBS library with the sequence ARRRRRRGATA. The 7 RBS from this collection that were submitted as BioBricks (Bba_K1351028-BBa_K1351034) are the ones that offer the best coverage of translation initiation rate space, number 1 being the optimal ''Bacillus'' RBS and number 7 being the weakest. <html> | + | Using the Salis lab's [https://salis.psu.edu/software/ RBS calculator] we calculated our RBS library with the sequence ARRRRRRGATA. The 7 RBS from this collection that were submitted as BioBricks ([http://parts.igem.org/Part:BBa_K1351028 BBa_K1351028]-[http://parts.igem.org/Part:BBa_K1351034 BBa_K1351034]) are the ones that offer the best coverage of translation initiation rate space, number 1 being the optimal ''Bacillus'' RBS and number 7 being the weakest. <html> |
| | </article> | | </article> |
| | </div> | | </div> |
| Line 228: |
Line 266: |
| | <article class="ac-small"> | | <article class="ac-small"> |
| | </html> | | </html> |
| - | Lorem ipsum dolor sit amet, consetetur sadipscing elitr, sed diam nonumy eirmod tempor invidunt ut labore et dolore magna aliquyam erat, sed diam voluptua. At vero eos et accusam et justo duo dolores et ea rebum. Stet clita kasd gubergren, no sea takimata sanctus est Lorem ipsum dolor sit amet. Lorem ipsum dolor sit amet, consetetur sadipscing elitr, sed diam nonumy eirmod tempor invidunt ut labore et dolore magna aliquyam erat, sed diam voluptua. At vero eos et accusam et justo duo dolores et ea rebum. Stet clita kasd gubergren, no sea takimata sanctus est Lorem ipsum dolor sit amet.
| + | The constructs have been created, the evaluation however is not yet finished. |
| | <html> | | <html> |
| | </article> | | </article> |
 "
"