Team:LMU-Munich/Application/Legal Issues
From 2014.igem.org
(→BaKillus as a diagnostic device) |
|||
| (5 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
{{Template:Team:LMU-Munich/Playground/menu}} | {{Template:Team:LMU-Munich/Playground/menu}} | ||
| - | + | <html> | |
| + | <div id="application-legal-issues-teaser" class="full-width teaser-img"> </div> | ||
| + | </html> | ||
= BaKillus as a medical product = | = BaKillus as a medical product = | ||
== Research and Development== | == Research and Development== | ||
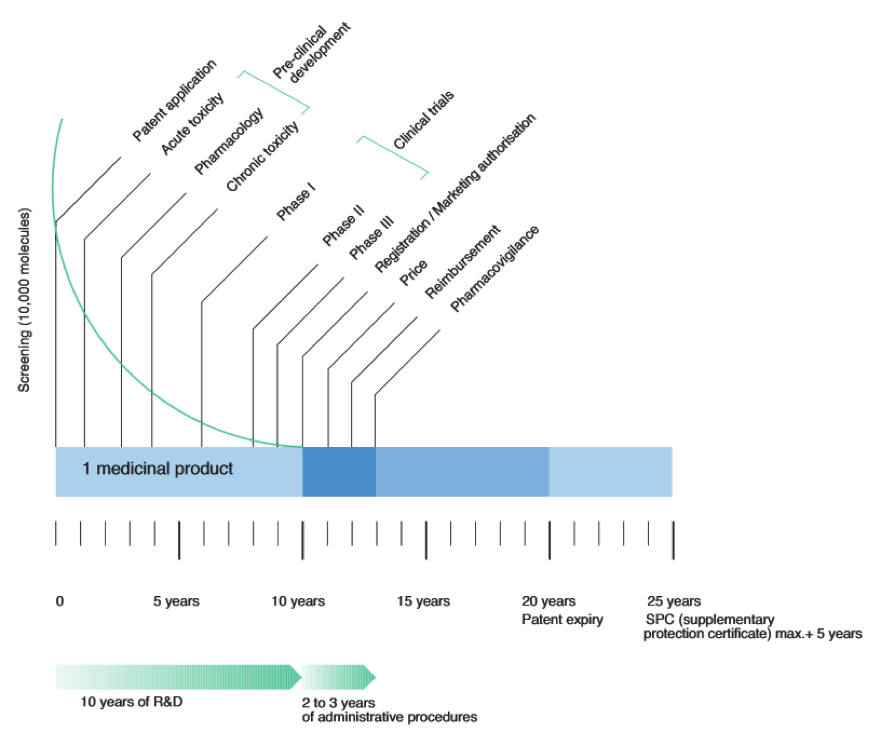
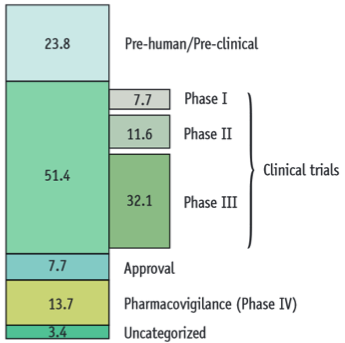
| - | BaKillus is intended to be used as a medical product in the treatment or prevention of MRSA infections. Hence, it is strictly regulated by national laws to protect and promote public health. Like every new drug, it would first have to be approved by the national regulatory agency by fulfilling certain requirements during the research and development (R & D) process. Generally, the process can be divided up into three parts: pre-clinical development, clinical trials and registration (Fig. 1A). The costs for the R & D process of a new drug have been estimated to be around $ 1.5 billion [ | + | BaKillus is intended to be used as a medical product in the treatment or prevention of MRSA infections. Hence, it is strictly regulated by national laws to protect and promote public health. Like every new drug, it would first have to be approved by the national regulatory agency by fulfilling certain requirements during the research and development (R & D) process. Generally, the process can be divided up into three parts: pre-clinical development, clinical trials and registration (Fig. 1A). The costs for the R & D process of a new drug have been estimated to be around $ 1.5 billion [https://www.ohe.org/publications/rd-cost-new-medicine], where the major costs (>50%) account for the clinical trials (Fig. 1B). To guarantee funding, development of a promising drug candidate typically starts with a patent application. Depending on the product and the desired market, different authorities are responsible for the approval procedure. |
[[File:LMU14 LegalIssues Timeline.png|center|thumb|800px|Fig. 1A: Typical time frame for the development of new drug applications [http://www.efpia.eu/uploads/Figures_2014_Final.pdf]]] | [[File:LMU14 LegalIssues Timeline.png|center|thumb|800px|Fig. 1A: Typical time frame for the development of new drug applications [http://www.efpia.eu/uploads/Figures_2014_Final.pdf]]] | ||
| Line 13: | Line 15: | ||
== Authorities == | == Authorities == | ||
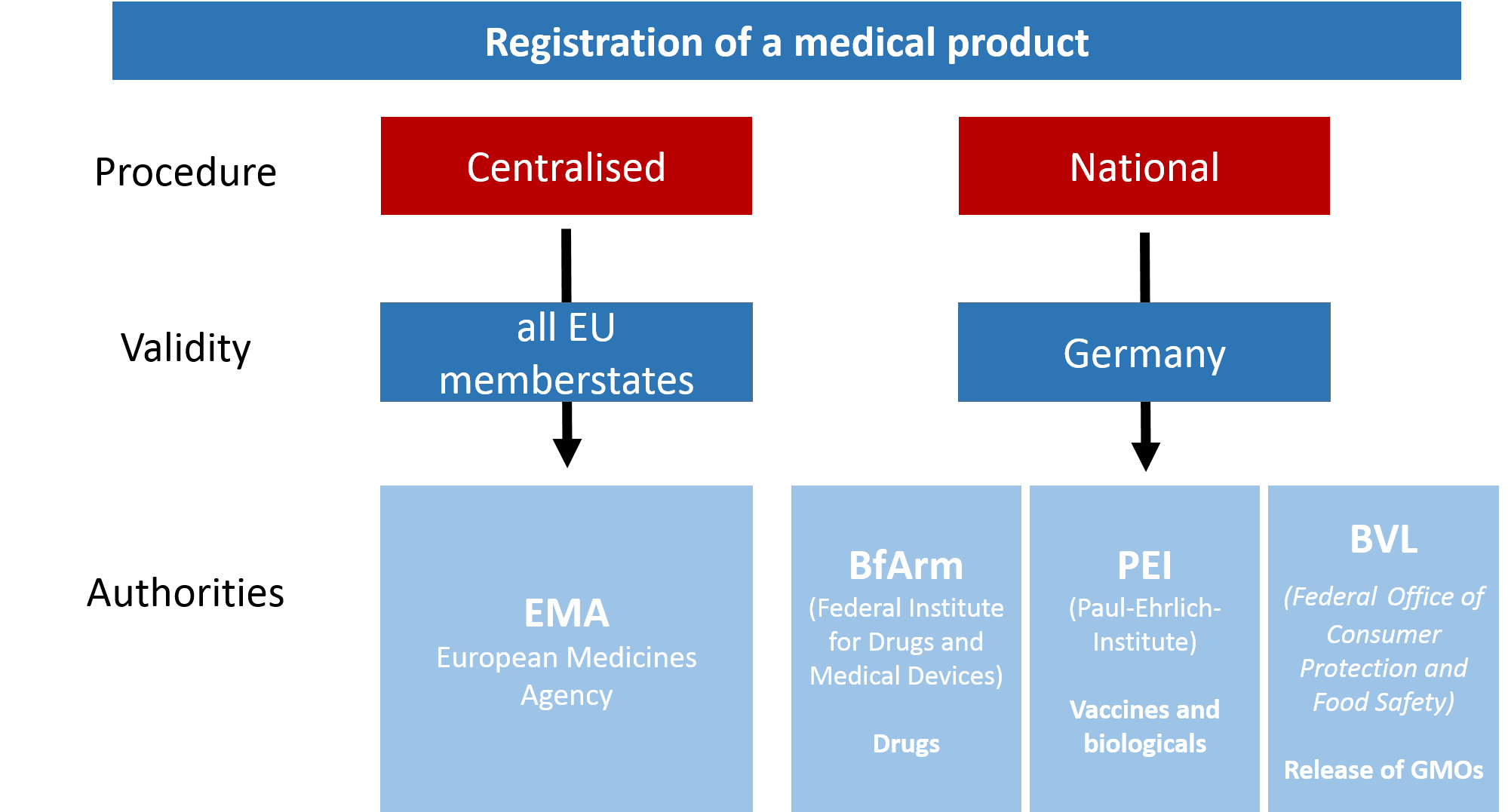
| - | To get the marketing authorisation for a new medical product in Germany, there are two possibilities: either a centralised procedure via the European Union (EU) or a national procedure, submitting the application to German authorities. However, for some types of medicinal products the centralised procedure is compulsory. This is the case for BaKillus, since it contains cells and is a product for advanced therapies (see below) [ | + | To get the marketing authorisation for a new medical product in Germany, there are two possibilities: either a centralised procedure via the European Union (EU) or a national procedure, submitting the application to German authorities. However, for some types of medicinal products the centralised procedure is compulsory. This is the case for BaKillus, since it contains cells and is a product for advanced therapies (see below) [http://www.pei.de/EN/institute/duties/duties-node.html]. For the centralised procedure the application has to be submitted to the European Medicines Agency (EMA), being simultaneously valid in all EU member states whilst validated by committees with representatives from each state (Fig. 2). The Committee for Advanced Therapies (CAT), one of several committees of the EMA, would most probably be responsible for our project. In Germany there are three major institutions responsible for market authorisation depending on the product: the BfARM, being responsible for chemical medicinal products, the PEI, responsible for vaccines and biologicals, and the BVL, responsible for veterinary medical products and the release of GMOs(Fig. 2). Due to its expertise with biologicals, the PEI is represented in the CAT amongst others. |
The general situation in the USA is mostly similar to the European/German system. Authority is given to the Federal Drug Administration (FDA) by the Federal Food, Drug, and Cosmetic Act (1938) (FD&C) which is a centralized institution in order to guarantee drug quality and safety. | The general situation in the USA is mostly similar to the European/German system. Authority is given to the Federal Drug Administration (FDA) by the Federal Food, Drug, and Cosmetic Act (1938) (FD&C) which is a centralized institution in order to guarantee drug quality and safety. | ||
| - | [[File: | + | [[File:LMU14_Authorities.png|center|thumb|600px|Fig. 2: Institutions responsible for registration on centralized and decentralized procedure]] |
| - | + | ||
---- | ---- | ||
| Line 23: | Line 24: | ||
== Legal directives affecting BaKillus == | == Legal directives affecting BaKillus == | ||
| - | According to the EU-directive 2001/83/EG Appendix I Part IV, BaKillus is a medicine for new therapies (“Arzneimittel für neuartige Therapien”) because it contains biologically altered cells as the active component for the therapeutic purpose (“biologische zu therapeutischen Zwecken veränderten Zellen als Wirkstoffe oder Wirkstoffbestandteile”). The medicines for new therapies are divided into two groups: gene therapeutics (“Gentherapeutika”) and somatic cell-therapeutics (“Somatische Zelltherapeutika”), with BaKillus belonging to the latter one. Although being covered up to this point by the directive, a subsequent paragraph (Part IV 2.) further classifies three types of somatic-cell therapeutics: autolog (from same patient), allogen (from another human) and xenogen (animal) cell-therapeutics. Although the term “animal” is very unprecisely defined, it probably applies in its broadest meaning to the class of Metazoa, if not just to Mammalia. Thus, in its current form the directive does not consider microbials as a medical treatment. This not only represents an incomplete status with regard to future treatments, but is even insufficient for current applications, since microbials are already used e.g. in fecal transplants [ | + | According to the EU-directive 2001/83/EG Appendix I Part IV, BaKillus is a medicine for new therapies (“Arzneimittel für neuartige Therapien”) because it contains biologically altered cells as the active component for the therapeutic purpose (“biologische zu therapeutischen Zwecken veränderten Zellen als Wirkstoffe oder Wirkstoffbestandteile”). The medicines for new therapies are divided into two groups: gene therapeutics (“Gentherapeutika”) and somatic cell-therapeutics (“Somatische Zelltherapeutika”), with BaKillus belonging to the latter one. Although being covered up to this point by the directive, a subsequent paragraph (Part IV 2.) further classifies three types of somatic-cell therapeutics: autolog (from same patient), allogen (from another human) and xenogen (animal) cell-therapeutics. Although the term “animal” is very unprecisely defined, it probably applies in its broadest meaning to the class of Metazoa, if not just to Mammalia. Thus, in its current form the directive does not consider microbials as a medical treatment. This not only represents an incomplete status with regard to future treatments, but is even insufficient for current applications, since microbials are already used e.g. in fecal transplants [http://www.economist.com/news/science-and-technology/21565586-bacterial-medicine-starting-emerge-bugs-system]. Thus, an update of the directive for engineered microorganisms would be important to account for this new class of therapeutics. |
| - | In the USA, the FDA has made first approaches towards creating guidances for innovative pharmaceutical products to enter the market. For instance, a draft guidance for sponsors has been published which deals with alternative therapies of fecal microbiota transplants against infections of ''Clostridium difficile'' that are not responsive to standard treatment. As BaKillus has a similar intention of treatment, such legislation would benefit our product. [ | + | In the USA, the FDA has made first approaches towards creating guidances for innovative pharmaceutical products to enter the market. For instance, a draft guidance for sponsors has been published which deals with alternative therapies of fecal microbiota transplants against infections of ''Clostridium difficile'' that are not responsive to standard treatment. As BaKillus has a similar intention of treatment, such legislation would benefit our product. [http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Vaccines/ucm387023.htm] Additionally an approach has been made targeting viral or bacterial-based gene therapy. [http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/CellularandGeneTherapy/ucm404050.htm] Furthermore, with respect to the release of microbial strains into the environment, a new draft guidance has been published by the FDA. Remarkably, the current design of BaKillus would fulfil most of the criteria listed therein .[http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/CellularandGeneTherapy/ucm401869.htm] |
| - | |||
== BaKillus as a diagnostic device == | == BaKillus as a diagnostic device == | ||
| - | Besides its primary use as a medical product, BaKillus can also be applied as a [https://2014.igem.org/Team:LMU-Munich/Application/Diagnosis_Treatment diagnosis tool]. In this case, it would be a medical device and thus no longer affected by the drug law. Since the device is designed as a closed system there would not be any contact between BaKillus and the environment. For this reason a request for release into the environment would not be necessary, according to the law on genetic engineering. For placing the BaKillus diagnosis device on the (European) market it would first have to be tested in the laboratory and fulfill all requirements of the ''in-vitro'' Diagnostic Directive (IVDD) to receive the so-called CE-certification. Unfortunately the | + | Besides its primary use as a medical product, BaKillus can also be applied as a [https://2014.igem.org/Team:LMU-Munich/Application/Diagnosis_Treatment diagnosis tool]. In this case, it would be a medical device and thus no longer affected by the drug law. Since the device is designed as a closed system there would not be any contact between BaKillus and the environment. For this reason a request for release into the environment would not be necessary, according to the law on genetic engineering. For placing the BaKillus diagnosis device on the (European) market it would first have to be tested in the laboratory and fulfill all requirements of the ''in-vitro'' Diagnostic Directive (IVDD) to receive the so-called CE-certification. Unfortunately the criteria for placing genetically modified organisms (GMOs) on the market are primarily designed for plants and not for bacteria. It must be assumed that the IVDD will be updated in the near future since there already have been alterations in the Directive 2001/18/EC Article 11 regarding the release of GMOs other than higher plants. |
Latest revision as of 13:53, 10 December 2014
BaKillus as a medical product
Research and Development
BaKillus is intended to be used as a medical product in the treatment or prevention of MRSA infections. Hence, it is strictly regulated by national laws to protect and promote public health. Like every new drug, it would first have to be approved by the national regulatory agency by fulfilling certain requirements during the research and development (R & D) process. Generally, the process can be divided up into three parts: pre-clinical development, clinical trials and registration (Fig. 1A). The costs for the R & D process of a new drug have been estimated to be around $ 1.5 billion [1], where the major costs (>50%) account for the clinical trials (Fig. 1B). To guarantee funding, development of a promising drug candidate typically starts with a patent application. Depending on the product and the desired market, different authorities are responsible for the approval procedure.
Authorities
To get the marketing authorisation for a new medical product in Germany, there are two possibilities: either a centralised procedure via the European Union (EU) or a national procedure, submitting the application to German authorities. However, for some types of medicinal products the centralised procedure is compulsory. This is the case for BaKillus, since it contains cells and is a product for advanced therapies (see below) [http://www.pei.de/EN/institute/duties/duties-node.html]. For the centralised procedure the application has to be submitted to the European Medicines Agency (EMA), being simultaneously valid in all EU member states whilst validated by committees with representatives from each state (Fig. 2). The Committee for Advanced Therapies (CAT), one of several committees of the EMA, would most probably be responsible for our project. In Germany there are three major institutions responsible for market authorisation depending on the product: the BfARM, being responsible for chemical medicinal products, the PEI, responsible for vaccines and biologicals, and the BVL, responsible for veterinary medical products and the release of GMOs(Fig. 2). Due to its expertise with biologicals, the PEI is represented in the CAT amongst others. The general situation in the USA is mostly similar to the European/German system. Authority is given to the Federal Drug Administration (FDA) by the Federal Food, Drug, and Cosmetic Act (1938) (FD&C) which is a centralized institution in order to guarantee drug quality and safety.
Legal directives affecting BaKillus
According to the EU-directive 2001/83/EG Appendix I Part IV, BaKillus is a medicine for new therapies (“Arzneimittel für neuartige Therapien”) because it contains biologically altered cells as the active component for the therapeutic purpose (“biologische zu therapeutischen Zwecken veränderten Zellen als Wirkstoffe oder Wirkstoffbestandteile”). The medicines for new therapies are divided into two groups: gene therapeutics (“Gentherapeutika”) and somatic cell-therapeutics (“Somatische Zelltherapeutika”), with BaKillus belonging to the latter one. Although being covered up to this point by the directive, a subsequent paragraph (Part IV 2.) further classifies three types of somatic-cell therapeutics: autolog (from same patient), allogen (from another human) and xenogen (animal) cell-therapeutics. Although the term “animal” is very unprecisely defined, it probably applies in its broadest meaning to the class of Metazoa, if not just to Mammalia. Thus, in its current form the directive does not consider microbials as a medical treatment. This not only represents an incomplete status with regard to future treatments, but is even insufficient for current applications, since microbials are already used e.g. in fecal transplants [http://www.economist.com/news/science-and-technology/21565586-bacterial-medicine-starting-emerge-bugs-system]. Thus, an update of the directive for engineered microorganisms would be important to account for this new class of therapeutics. In the USA, the FDA has made first approaches towards creating guidances for innovative pharmaceutical products to enter the market. For instance, a draft guidance for sponsors has been published which deals with alternative therapies of fecal microbiota transplants against infections of Clostridium difficile that are not responsive to standard treatment. As BaKillus has a similar intention of treatment, such legislation would benefit our product. [http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Vaccines/ucm387023.htm] Additionally an approach has been made targeting viral or bacterial-based gene therapy. [http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/CellularandGeneTherapy/ucm404050.htm] Furthermore, with respect to the release of microbial strains into the environment, a new draft guidance has been published by the FDA. Remarkably, the current design of BaKillus would fulfil most of the criteria listed therein .[http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/CellularandGeneTherapy/ucm401869.htm]
BaKillus as a diagnostic device
Besides its primary use as a medical product, BaKillus can also be applied as a diagnosis tool. In this case, it would be a medical device and thus no longer affected by the drug law. Since the device is designed as a closed system there would not be any contact between BaKillus and the environment. For this reason a request for release into the environment would not be necessary, according to the law on genetic engineering. For placing the BaKillus diagnosis device on the (European) market it would first have to be tested in the laboratory and fulfill all requirements of the in-vitro Diagnostic Directive (IVDD) to receive the so-called CE-certification. Unfortunately the criteria for placing genetically modified organisms (GMOs) on the market are primarily designed for plants and not for bacteria. It must be assumed that the IVDD will be updated in the near future since there already have been alterations in the Directive 2001/18/EC Article 11 regarding the release of GMOs other than higher plants.

Hi there!
Welcome to our Wiki! I'm BaKillus, the pathogen-hunting microbe, and I'll guide you on this tour through our project. If you want to learn more about a specific step, you can simply close the tour and come back to it anytime you like. So let's start!

What's the problem?
First of all, what am I doing here? The problem is, pathogenic bacteria all around the world are becoming more and more resistant against antimicrobial drugs. One major reason for the trend is the inappropriate use of drugs. With my BaKillus super powers, I want to reduce this misuse and thus do my part to save global health.

Sensing of pathogens
To combat the pathogenic bacteria, I simply eavesdrop on their communication. Bacteria talk with each other via quorum sensing systems, which I use to detect them and trigger my responses.

Adhesion
The more specific and effective I can use my powers, the lower the danger is of provoking new resistance development. So I catch pathogens whenever I get hold of them and stick to them until my work is done.

Killing
Talking about my work - killing pathogens is finally what I am made for. In response to quorum sensing molecules of the pathogens, I export a range of antimicrobial substances leading to dissipation of biofilms and the killing of the targeted bacteria.

Suicide switch
When the job is done and all the bad guys are finished, you don't need a super hero anymore. So after fulfilling my work I say goodbye to the world by activating my suicide switch.

Application
Of course I'm not only a fictional hero, but a very real one. In two different prototypes, I could be used for diagnosis or treatment of pathogen-caused diseases. However, there is still a whole lot of regulational and economical questions that have to be answered before.

See you!
So now you know my short story - and it is time for me to return to my fight for a safer world. Feel free to take a closer look on my super powers, the process of my development or the plans for a medical application.
 "
"