Team:Freiburg/Content/Results/Light system
From 2014.igem.org
| Line 11: | Line 11: | ||
<p>Light inducible systems allow spatial and temporal control over gene expression. Our goal was to express the mCAT-1 receptor through a light inducible system to allow spatiotemporal control of the virus entry into the target cells. We used two different systems, the blue light system based on the LOV domain from Avena sativa, and the red light system from Arabidopsis thaliana. | <p>Light inducible systems allow spatial and temporal control over gene expression. Our goal was to express the mCAT-1 receptor through a light inducible system to allow spatiotemporal control of the virus entry into the target cells. We used two different systems, the blue light system based on the LOV domain from Avena sativa, and the red light system from Arabidopsis thaliana. | ||
| - | The expression of the receptor in non-murine cell lines by illumination | + | |
| + | |||
| + | After | ||
| + | The expression of the receptor in non-murine cell lines is achieved by illumination with light of the appropriate wavelength. Since the viral vector only infect cells that express the mCAT-1 receptor on the plasma membrane, our goal was transfered genes into distinct areas of an otherwise homogeneous cell layer . | ||
</p> | </p> | ||
Revision as of 21:18, 17 October 2014
The Light System
Light inducible systems allow spatial and temporal control over gene expression. Our goal was to express the mCAT-1 receptor through a light inducible system to allow spatiotemporal control of the virus entry into the target cells. We used two different systems, the blue light system based on the LOV domain from Avena sativa, and the red light system from Arabidopsis thaliana. After The expression of the receptor in non-murine cell lines is achieved by illumination with light of the appropriate wavelength. Since the viral vector only infect cells that express the mCAT-1 receptor on the plasma membrane, our goal was transfered genes into distinct areas of an otherwise homogeneous cell layer .
Function Of The Light System
As the function of the light system, we use, is really important for the function of our whole system, we tested two different light systems. The red light system is based on based on the Arabidopsis thaliana protein phytochrome B (PhyB) that binds to the phytochrome-interacting factor 6 (PIF6) after illumination with red light (660 nm), whereas the blue light system contains a domain from Avena sativa (AsLOV2) that is assessible to other parts of the system upon illumination with blue light (465 nm).
Since, it is known that the red light system works better in chinese hamster ovary (CHO) cells, we used this cell line to test the function of this light system. In order to investigate the functionality of the blue light system, we tested both, CHO cells and human embryonic kidney (HEK293) cells. As reporter the gene seap was transfected together with the genes for the light systems (red light system: PKM006, PKM078; blue light system: PKM292, PKM297, PKM084). A SEAP-assay was performed after illumination. Regarding SEAP concentrations in the supernatant of each sample two important findings were archived. First, the blue light system works much more efficient than the red light system; and the blue light system works better with HEK cells than with CHO cells (Fig.1).

Figure 1: Comparing the blue light system in CHO cells and in HEK293 cells and comparison to the red light system in CHO cells.
To test the efficiency of the light system light induced SEAP was used as a marker. A SEAP assay was performed after 24 hours of incubation. Labjournal

Figure 2: Efficiency of the blue light system using different durations of illumination.
To test the efficiency of the light system light induced SEAP was used as a marker. A SEAP assay was performed after 24 hours of incubation. Cells were incubated with blue light for 1 hour, 2.5 hours and 5 hours. Labjournal
The blue light system is very effective in activating an existing reporter, like the gene for seap, brought into the cell before illumination. As we know that the duration of illuminating the system with blue light (452 nm) is critical for its efficiency, we tested various time spans for illumination. Our results indicate that five hours of illumination lead to the highest activation level of SEAP expression (Fig.2).
Another important finding was the specificity of the blue light system. As we always have a dark control in our experiments, we determined almost no activation of the SEAP reporter in these samples leading to a very low background level in our system.
SEAP as Reporter
In order to test the capability of the blue light system for pattern generation, we transfected HEK293 cells with the appropriate plasmids (PKM292 and PKM297) using seap as a reporter gene (PKM084). Cells were transfected in suspension and seeded on 96 well plates afterwards. Single wells of these plates were covered with a photo mask that prevents illumination with blue light. Since, all other wells on the plate were exposed to the blue light, SEAP expression was induced in these wells. SEAP that was secreted into the culture medium leads to colour changing from red to yellow after addition of pNPP (chromogenic substrate) by its phosphorylation.
First we generated a coarse pattern (fig.) realizing that by scattered light also covered wells close to illuminated wells were exposed to light. This was leading to an unclear pattern. However, we managed to give an idea of the desired pattern (Fig.3, A-B).
Since, it was essential to completely prevent light exposure on covered plates we tested 96 well plates with optical separated wells. As shown in fig. we could generate patterns with sharp contours (Fig.3, C-D).
Colour changing of pNPP in cell culture medium could be measured at 405 nm in a plate reader. Calculating the gradient of light absorption we also generated patterns using a data matrix (Excel) by setting a distinct threshold, thus making pattern recognition easier.
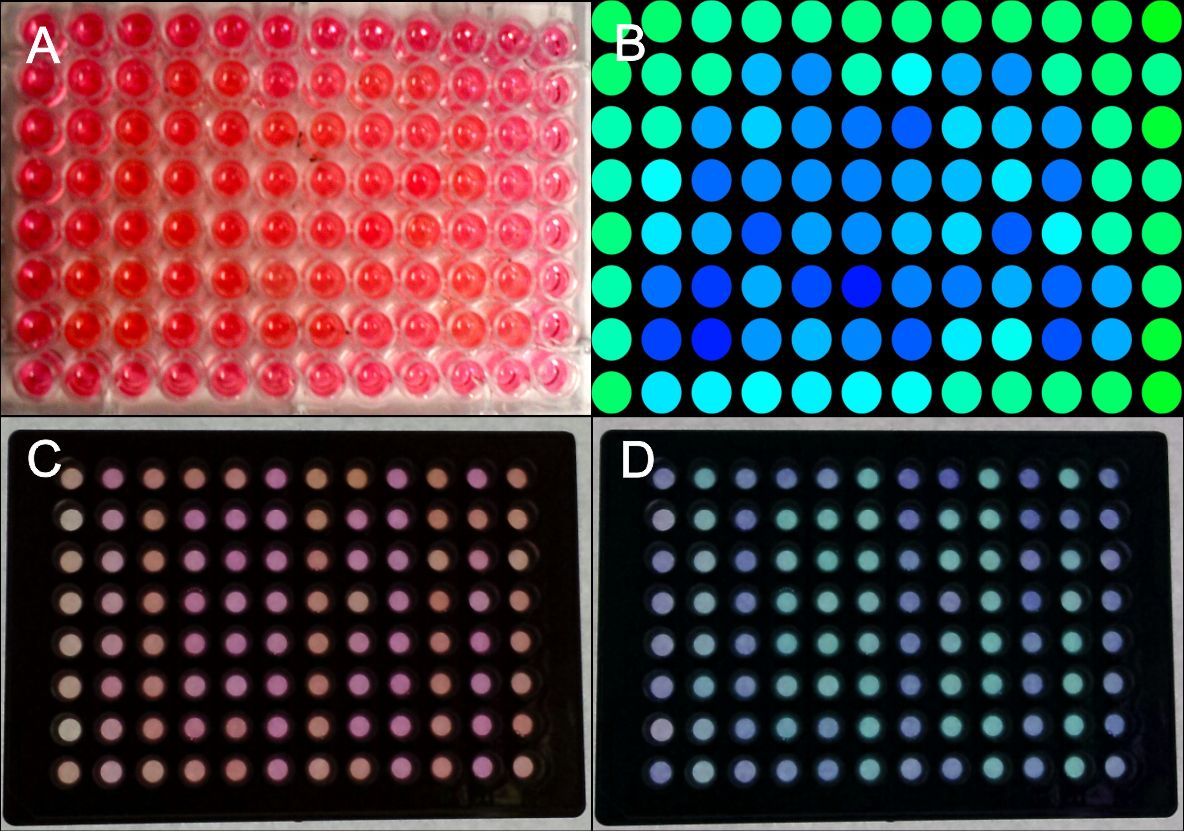
Figure 3: Generation of patterns on 96W plates.
HEK293 cells were transfected with the blue light system (PKM292, PKM297) and light induced SEAP as reporter (PKM084). Single wells were covered with a photo mask to prevent cell from light exposure. 24 hours after illumination with blue light a SEAP assay was performed leading to colour changing of wells containing SEAP. (A) Photo mask in heart form, (B) a pink colour was set to green and a yellow colour was set to blue at a computer, (C) the same experiment was performed with multiple well plates with separated wells, (D) the contrast an the colours of the wells were changed at a computer. All wells together are forming the word: IGEM. Labjournal 1 Labjournal 2
For generating QR codes with our method 384W plates were used. Since we had a collaboration with the iGEM Team Aachen 2014, they made a photo mask for a QR code for us fitting on these plates (Fig.4) Collaborations.
Figure 4: Generation of a QR code on a 384 well plate. HEK293 cells were transfected with the blue light system (PKM292, PKM297) and light induced SEAP as reporter (PKM084). Single wells were covered with a photo mask to prevent cell from light exposure using a photo mask for a QR code (made by iGEM Team Aachen 2014). 24 hours after illumination with blue light a SEAP assay was performed leading to colour changing of wells containing SEAP. Only a part of the plate was used. (A) Photo mask for QR code generation, (B) Picture of the 384W plate after the SEAP assay, (C) the same plate with contrast of the colours increased at a computer.Labjournal
 "
"