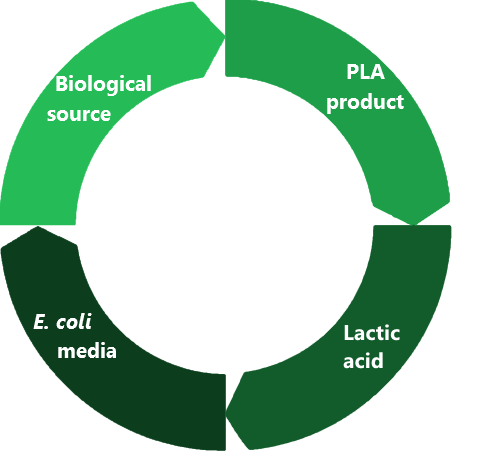
Team:RHIT/Sustainability
From 2014.igem.org
| (11 intermediate revisions not shown) | |||
| Line 7: | Line 7: | ||
</style> | </style> | ||
| + | <style> | ||
| + | html { | ||
| + | background-color:#DFFFE9; | ||
| + | } | ||
| + | |||
| + | #content { | ||
| + | background-color:#DFFFE9; | ||
| + | } | ||
| + | |||
| + | #bodyContent { | ||
| + | background-color:#DFFFE9; | ||
| + | } | ||
| + | </style> | ||
<style type="text/css"> | <style type="text/css"> | ||
| Line 13: | Line 26: | ||
margin:0; | margin:0; | ||
width: 100%; | width: 100%; | ||
| - | background:# | + | background:#DFFFE9; |
} | } | ||
| Line 35: | Line 48: | ||
font-size:14px; | font-size:14px; | ||
line-height:14px; | line-height:14px; | ||
| - | background:# | + | background:#DFFFE9; |
text-shadow:1px 1px 1px rgba(255,255,255,0.3); | text-shadow:1px 1px 1px rgba(255,255,255,0.3); | ||
font-weight:700; | font-weight:700; | ||
| Line 138: | Line 151: | ||
</div> | </div> | ||
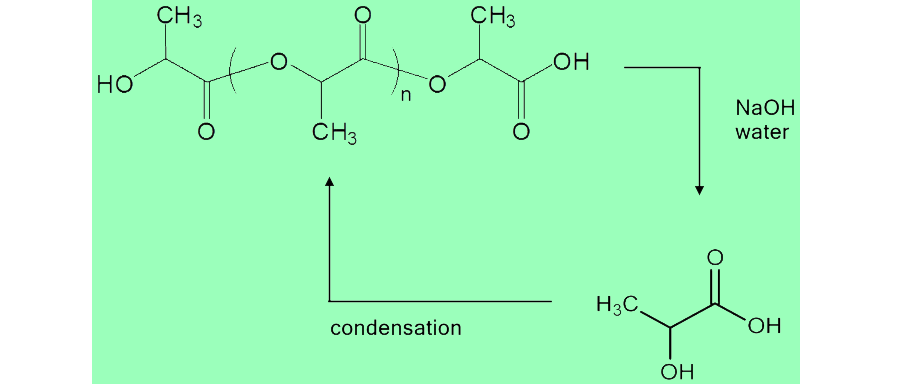
| - | <div style="margin:10px 250px 100px | + | <div style="margin:10px 250px 100px 250px"> |
<img src="http://i1265.photobucket.com/albums/jj502/bauhand/PLAreaction_zps5c13ea5d.png" width="650px" align="right"/> | <img src="http://i1265.photobucket.com/albums/jj502/bauhand/PLAreaction_zps5c13ea5d.png" width="650px" align="right"/> | ||
| Line 203: | Line 216: | ||
<label for="checkbox-3">LDH Assay</label> | <label for="checkbox-3">LDH Assay</label> | ||
<div class="content"> | <div class="content"> | ||
| - | <p> | + | <p><p>Run a test assay using known concentrations of lactate.</p> |
| - | </p> | + | <p>Make up the following stock solutions:</p> |
| + | <p><u>Lactate Stock (120mM):</u></p> | ||
| + | <ul> | ||
| + | <li>.57606g lactate</li> | ||
| + | <li>50 mL 10mM Tris-HCl (pH 8.6)</li> | ||
| + | </ul> | ||
| + | <p><u>NAD+ Stock (12mM):</u></p> | ||
| + | <ul> | ||
| + | <li>.0398g NAD+</li> | ||
| + | <li>5 mL 10mM Tris-HCl (pH 8.6)</li> | ||
| + | </ul> | ||
| + | <p><u>Bicarbonate Stock (18mM):</u> | ||
| + | <ul> | ||
| + | <li>.00302g NaHCO<sub>3</sub></li> | ||
| + | <li>2 mL 10mM Tris-HCl (pH 8.6)</li> | ||
| + | </ul> | ||
| + | <p>Using the lactate stock solution, make up the following dilutions: 1mM, .5mM, .2mM, .1mM, 0mM</p> | ||
| + | <p>In a cuvette, add 0.6 mL 1mM dilution, 0.4 mL NAD+ stock, and 0.2 mL Bicarbonate stock. Place the cuvette in the spectrophotometer and measure the absorbance. Leaving the cuvette in the spectrophotometer, add 10 µL lactate dehydrogenase (LDH) enzyme. Allow the reaction to run in the cuvette until the absorbance plateaus. Repeat procedure with remaining dilutions in separate cuvettes.</p> | ||
| + | <p>The following is the data from the test run:</p> | ||
| + | <table> | ||
| + | <tr style="background-color:#DFFFE9; border:1px solid black"> | ||
| + | <th style="border:1px solid black">Concentrations</th> | ||
| + | <th style="border:1px solid black">OD Before LDH</th> | ||
| + | <th style="border:1px solid black">OD After LDH</th> | ||
| + | <th style="border:1px solid black">Difference</th> | ||
| + | </tr> | ||
| + | |||
| + | <tr style="background-color:#DFFFE9; border:1px solid black"> | ||
| + | <td style="border:1px solid black">0 mM</td> | ||
| + | <td style="border:1px solid black">0.242</td> | ||
| + | <td style="border:1px solid black">0.242</td> | ||
| + | <td style="border:1px solid black">0</td> | ||
| + | </tr> | ||
| + | |||
| + | <tr style="background-color:#DFFFE9; border:1px solid black"> | ||
| + | <td style="border:1px solid black">.1 mM</td> | ||
| + | <td style="border:1px solid black">0.284</td> | ||
| + | <td style="border:1px solid black">0.367</td> | ||
| + | <td style="border:1px solid black">0.083</td> | ||
| + | </tr> | ||
| + | |||
| + | <tr style="background-color:#DFFFE9; border:1px solid black"> | ||
| + | <td style="border:1px solid black">.2 mM</td> | ||
| + | <td style="border:1px solid black">0.299</td> | ||
| + | <td style="border:1px solid black">0.401</td> | ||
| + | <td style="border:1px solid black">0.102</td> | ||
| + | </tr> | ||
| + | |||
| + | <tr style="background-color:#DFFFE9; border:1px solid black"> | ||
| + | <td style="border:1px solid black">.5 mM</td> | ||
| + | <td style="border:1px solid black">0.31</td> | ||
| + | <td style="border:1px solid black">0.454</td> | ||
| + | <td style="border:1px solid black">0.144</td> | ||
| + | </tr> | ||
| + | |||
| + | <tr style="background-color:#DFFFE9; border:1px solid black"> | ||
| + | <td style="border:1px solid black">1 mM</td> | ||
| + | <td style="border:1px solid black">0.244</td> | ||
| + | <td style="border:1px solid black">0.483</td> | ||
| + | <td style="border:1px solid black">0.239</td> | ||
| + | </tr> | ||
| + | |||
| + | <tr style="background-color:#DFFFE9; border:1px"> | ||
| + | <td style="border:1px solid black">1 mM</td> | ||
| + | <td style="border:1px solid black">0.297</td> | ||
| + | <td style="border:1px solid black">0.54</td> | ||
| + | <td style="border:1px solid black">0.243</td> | ||
| + | </tr> | ||
| + | </table> | ||
| + | |||
| + | |||
| + | </p><br> | ||
| + | <p>The same protocol was replicated using the lactic acid solution obtained from PLA degradation and purification. Dilutions were made until the absorbance spectrum showed a useful peak at 340 nm. This dilution was then diluted by a factor of two to obtain further data. The assay will be run again to collect more data points before analysis. | ||
</div> | </div> | ||
</li> | </li> | ||
Latest revision as of 02:10, 17 October 2014
 "
"