Team:Bielefeld-CeBiTec/Project/CO2-fixation/Carboxysome
From 2014.igem.org
| Line 83: | Line 83: | ||
<br> | <br> | ||
Generally, there are two different types of CBs found, called α-type and β-type carboxysomes. This classification is based on the differences in their component proteins and organisation of their corresponding genes (<a href="#badger2002 (b)">Batger et al., 2002</a>). | Generally, there are two different types of CBs found, called α-type and β-type carboxysomes. This classification is based on the differences in their component proteins and organisation of their corresponding genes (<a href="#badger2002 (b)">Batger et al., 2002</a>). | ||
| - | α-type carboxysomes are found in α-cyanobacteria like some <i>Synechococcus</i> species or the chemoautotroph organism <i>Halothiobacillus neapolitanus</i>. This type of carboxysome contains the type I RuBisCo. Furthermore the respective genes for the carboxysome are arranged in a single operon. In β-type carboxysomes occurs the type II RuBisCo, containing no small subunits. In contrast to α-type carboxysomes, the genes are localisated in multiple gene clusters. (<a href="#badger2002 (b)">Batger et al., 2002</a>; <a href="#bonacci2011">Bonacci et al., 2011</a>; <a href="#yeates2008">Yeates et al., 2008</a>) A possible difference in functionality between both types of carboxysomes is not fully understood (<a href="#yeates2008">Yeates et al., 2008</a>). | + | α-type carboxysomes are found in α-cyanobacteria like some <i>Synechococcus</i> species or the chemoautotroph organism <a href="https://2014.igem.org/Team:Bielefeld-CeBiTec/Notebook/Organisms#H.neapolitanus" target="_blank"><i>Halothiobacillus neapolitanus</i></a>. This type of carboxysome contains the type I RuBisCo. Furthermore the respective genes for the carboxysome are arranged in a single operon. In β-type carboxysomes occurs the type II RuBisCo, containing no small subunits. In contrast to α-type carboxysomes, the genes are localisated in multiple gene clusters. (<a href="#badger2002 (b)">Batger et al., 2002</a>; <a href="#bonacci2011">Bonacci et al., 2011</a>; <a href="#yeates2008">Yeates et al., 2008</a>) A possible difference in functionality between both types of carboxysomes is not fully understood (<a href="#yeates2008">Yeates et al., 2008</a>). |
<br> | <br> | ||
<br> | <br> | ||
| Line 89: | Line 89: | ||
<br> | <br> | ||
<br> | <br> | ||
| - | <i>Halothiobacillus neapolitanus</i> is a model organism for the carboxysome. This organism is a Gram negative proteobacterium which belongs to the purple sulfur bacteria. It is obligate aerob. <i>H. neapolitanus</i> is know to tolerate and metabolize high amounts of sulfides. | + | <a href="https://2014.igem.org/Team:Bielefeld-CeBiTec/Notebook/Organisms#H.neapolitanus" target="_blank"><i>Halothiobacillus neapolitanus</i></a> is a model organism for the carboxysome. This organism is a Gram negative proteobacterium which belongs to the purple sulfur bacteria. It is obligate aerob. <a href="https://2014.igem.org/Team:Bielefeld-CeBiTec/Notebook/Organisms#H.neapolitanus" target="_blank"><i>H. neapolitanus</i></a> is know to tolerate and metabolize high amounts of sulfides. |
| - | <i>H. neapolitanus</i> is a chemolitoautotroph modell organism for the carboxysome with a diameter of 120nm. This type of carboxysome dominates in oligotrophic oceans (cso-carboxysome or alpha type). It has to be distinguished from the ccm-carboxysome found in several other marine- and freshwater cyanobacteria (beta type). | + | <a href="https://2014.igem.org/Team:Bielefeld-CeBiTec/Notebook/Organisms#H.neapolitanus" target="_blank"><i>H. neapolitanus</i></a> is a chemolitoautotroph modell organism for the carboxysome with a diameter of 120nm. This type of carboxysome dominates in oligotrophic oceans (cso-carboxysome or alpha type). It has to be distinguished from the ccm-carboxysome found in several other marine- and freshwater cyanobacteria (beta type). |
The first occurence was 1957 by Parker et al.Recent studies have been made on this organism (<a href="#bonacci2011">Bonacci et al., 2011</a>; <a href="#cannon1983">Cannon, Shively 1983</a>; <a href="#holthuijzen1986">Holthuijzen et al., 1986</a>; <a href="#so2004">So et al., 2004</a>). The native carboxysomes are α-type carboxysomes, the operon includes nine gene. One more gene, csoS1D was found, which is located outside of the core operon. (<a href="#bonacci2011">Bonacci et al., 2011</a>) A structural overview of the nine genes including the tenth gene is shown in Figure 2. | The first occurence was 1957 by Parker et al.Recent studies have been made on this organism (<a href="#bonacci2011">Bonacci et al., 2011</a>; <a href="#cannon1983">Cannon, Shively 1983</a>; <a href="#holthuijzen1986">Holthuijzen et al., 1986</a>; <a href="#so2004">So et al., 2004</a>). The native carboxysomes are α-type carboxysomes, the operon includes nine gene. One more gene, csoS1D was found, which is located outside of the core operon. (<a href="#bonacci2011">Bonacci et al., 2011</a>) A structural overview of the nine genes including the tenth gene is shown in Figure 2. | ||
| Line 96: | Line 96: | ||
<div class="element" style="width:850px; text-align:center"> | <div class="element" style="width:850px; text-align:center"> | ||
<a href="https://static.igem.org/mediawiki/2014/3/33/Bielefeld_CeBiTec_2014-09-24_csoS1-4_cPCR_08_22.png" target="_blank"><img src="https://static.igem.org/mediawiki/2014/8/83/Bielefeld-CeBiTec_14-10-16_carboxysome_operon.png" width="850"></a><br> | <a href="https://static.igem.org/mediawiki/2014/3/33/Bielefeld_CeBiTec_2014-09-24_csoS1-4_cPCR_08_22.png" target="_blank"><img src="https://static.igem.org/mediawiki/2014/8/83/Bielefeld-CeBiTec_14-10-16_carboxysome_operon.png" width="850"></a><br> | ||
| - | <font size="2"><b>Figure 2:</b> Schematic mapping of the caboxysomal gene of <i>Halothiobacillus neapolitanus</i>. csoS1D is also shwon here, although it is not found in the carboxysomal operon. </font> | + | <font size="2"><b>Figure 2:</b> Schematic mapping of the caboxysomal gene of <a href="https://2014.igem.org/Team:Bielefeld-CeBiTec/Notebook/Organisms#H.neapolitanus" target="_blank"><i>Halothiobacillus neapolitanus</i></a>. csoS1D is also shwon here, although it is not found in the carboxysomal operon. </font> |
</div> </center> | </div> </center> | ||
| Line 113: | Line 113: | ||
| - | <b>Table1:</b> Functions of the proteins coded in the carboxysomal operon of <i>Halothiobacillus neapolitanus</i> included CsoS1D. | + | <b>Table1:</b> Functions of the proteins coded in the carboxysomal operon of <a href="https://2014.igem.org/Team:Bielefeld-CeBiTec/Notebook/Organisms#H.neapolitanus" target="_blank"><i>Halothiobacillus neapolitanus</i></a> included CsoS1D. |
<table width="100%" border="1" cellpadding="5" style="background-color:transparent"> | <table width="100%" border="1" cellpadding="5" style="background-color:transparent"> | ||
<tr> | <tr> | ||
Revision as of 11:55, 16 October 2014
CO2 Fixation
Carboxysome
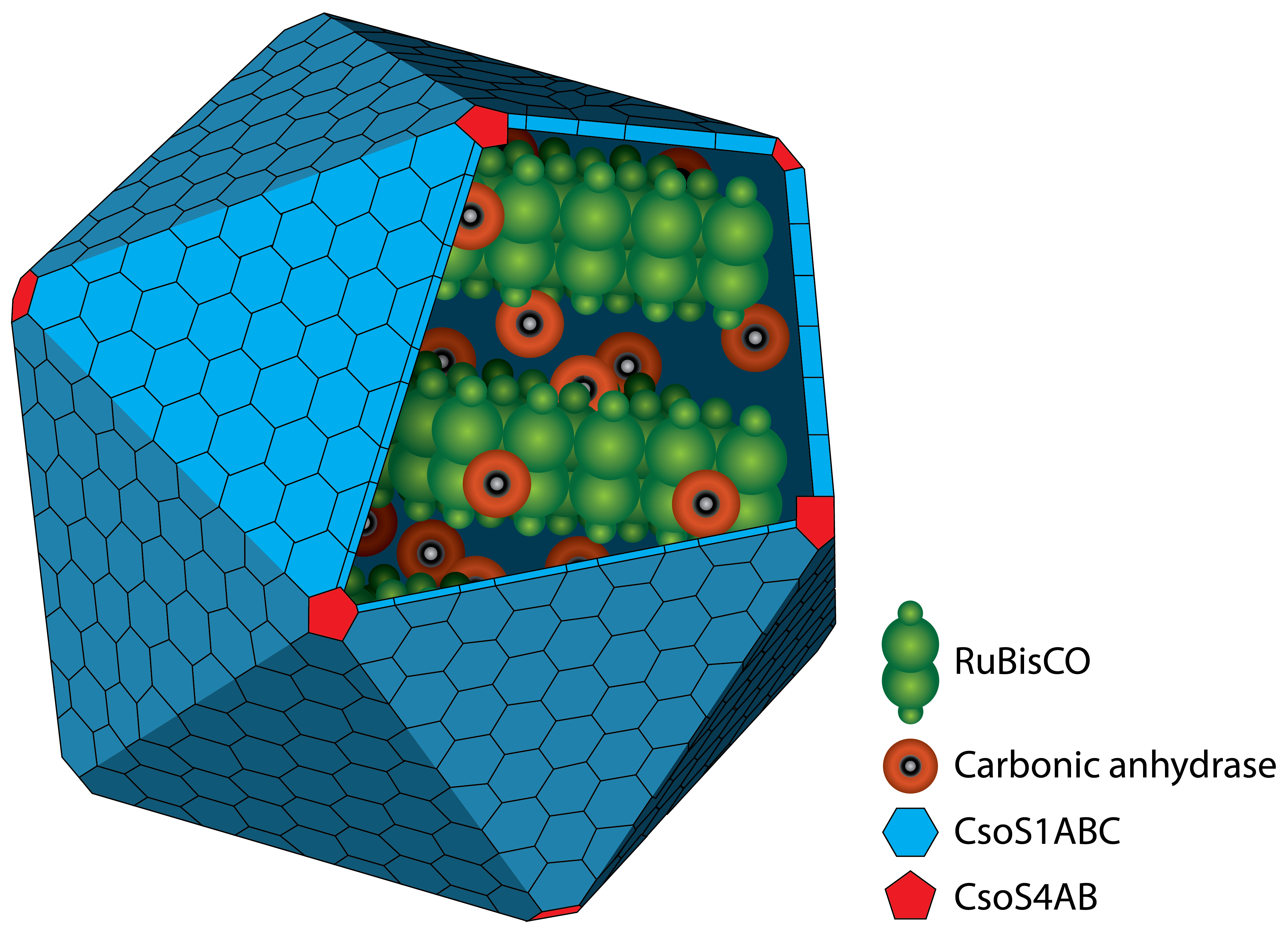
Figure 1: Schematic view of the Carboxysome. The RuBisCo and the carbonic anhydrase are surrounded by different carboxysomal shell proteins, which forms a defined structure. In the inside, carbon fixation takes places.
A carboxysome is a bacterial microcompartiment (BMC) surrounded by a protein shell. Carboxysomes (CB) play an essential role in carbon fixation mechanism, as they are the enzym-containing organelles for these metabolic pathways (Bonacci et al., 2011; Shively et al., 1973). The first carboxysomes were discovered in 1956, and purified carboxysomes were first characterized in 1973 by Shively et al., 1973.
Carboxysomes, as BMCs, are formed by different shell proteins, surrounding an enzyme-containing lumen, which is rigorous seperated from the cytoplasm. Thus, the possibility is given to enable reaction pathways incongruous with the conditions in the cytoplasm.
The protein shell consists of two different types of proteins. Pentamers are used for the vertices of the icosaeder and hexamers for the facets. The main constituent of the shell is the CsoS1A protein, which makes arround 13 % of the total protein mass. Carboxysomes are between 80 and 120 nm in diamter (Kehrfeld et al., 2005). In the interior, there are two different types of enzymes. On the one hand there is the
RuBisCo which catalyses the carboxylation or oxygenation of ribulose-1,5-bisphosphate. On the other hand there is the carbonic anhydrase which converts hydrogen carbonate (HCO3-) to carbon dioxide. The resulting carbon dioxide is the substrate for the RuBisCO. A schematic image of the carboxysome is given in Figure 1.
Generally, there are two different types of CBs found, called α-type and β-type carboxysomes. This classification is based on the differences in their component proteins and organisation of their corresponding genes (Batger et al., 2002).
α-type carboxysomes are found in α-cyanobacteria like some Synechococcus species or the chemoautotroph organism Halothiobacillus neapolitanus. This type of carboxysome contains the type I RuBisCo. Furthermore the respective genes for the carboxysome are arranged in a single operon. In β-type carboxysomes occurs the type II RuBisCo, containing no small subunits. In contrast to α-type carboxysomes, the genes are localisated in multiple gene clusters. (Batger et al., 2002; Bonacci et al., 2011; Yeates et al., 2008) A possible difference in functionality between both types of carboxysomes is not fully understood (Yeates et al., 2008).
The advantage of the microcompartiment is the concentration of carbon dioxide in its lumen. Carboxyomes play a central role in carbon concentrating mechanism (CCM) (Bonacci et al., 2011; Yeates et al., 2008). This mechanism is used for accumulation of CO2 in autotrophic prokaryotes. The advantage is that the concentration of carbon dioxide inside the carboxysome can be much higher than outside which increases the efficiency of the RuBisCO-catalyzed reaction. The carboxysome as a place of carbon dioxide fixation was first described in 1990 (Reinhold et al., 1990). The first reaction of the CCM is the transport of atmospheric carbon dioxide or hydrogencarbonate (HCO3-) into the cells. Accumulation of inorganic carbon is archieved by transmembrane pumps and transporters in the cell membrane. At physiological pH values (approximate 7), the balance is clearly on the side of hydrogencarbonate. Hydrogencarbonate is able to diffuse across the shell of the carboxysome. The carbonix anhydrase (CA), located inside the carboxysome, catalyses the conversion of bicarbonate to the substrate for RuBisCo, gaseous carbon dioxide. The CA is assosciated with the shell and presents only a small number of the carboxysome proteins. Thus, the CA, also named as CsoSCA or CSOS3, acts to saturate the lumen with carbon dioxide and enables high effective concentrations of CO2, so that the RuBisCo is able to work near its maximal reaction rate with high specifity. The formed product of the RuBisCo, 3-phosphoglycerate, is used in the metabolism of the cells. (Batger et al., 2002 (a); Bonacci et al., 2011; Yeates et al., 2008) Diffusive loss of carbon dioxide is propably provided by the outer shell membran acting as a barrier for the loss of CO2. How the shell is able to control the entry and exit of larger substrates is not fully understood (Yeates et al., 2008).
Halothiobacillus neapolitanus is a model organism for the carboxysome. This organism is a Gram negative proteobacterium which belongs to the purple sulfur bacteria. It is obligate aerob. H. neapolitanus is know to tolerate and metabolize high amounts of sulfides.
H. neapolitanus is a chemolitoautotroph modell organism for the carboxysome with a diameter of 120nm. This type of carboxysome dominates in oligotrophic oceans (cso-carboxysome or alpha type). It has to be distinguished from the ccm-carboxysome found in several other marine- and freshwater cyanobacteria (beta type).
The first occurence was 1957 by Parker et al.Recent studies have been made on this organism (Bonacci et al., 2011; Cannon, Shively 1983; Holthuijzen et al., 1986; So et al., 2004). The native carboxysomes are α-type carboxysomes, the operon includes nine gene. One more gene, csoS1D was found, which is located outside of the core operon. (Bonacci et al., 2011) A structural overview of the nine genes including the tenth gene is shown in Figure 2.
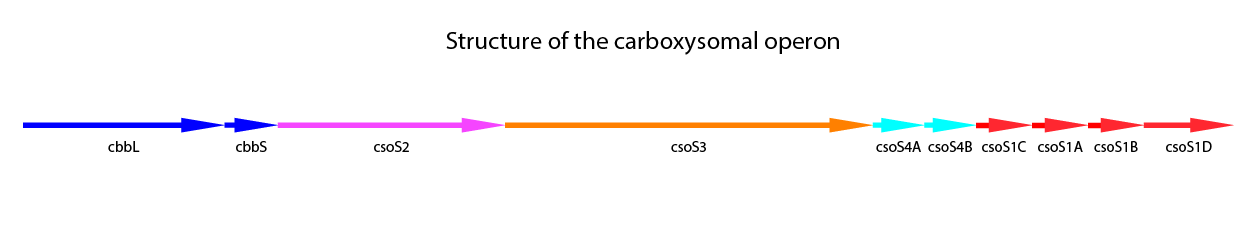
Figure 2: Schematic mapping of the caboxysomal gene of Halothiobacillus neapolitanus. csoS1D is also shwon here, although it is not found in the carboxysomal operon.
The further genes in the carboxysomal operon are related to the shell proteins. CsoS1A-C are the main shell proteins, building up the structure of the carboxysome. CsoS1A is responsible for the hexameric quaternary structure and is the major component of the CB shell. The gene csoS1D is not localized in the carboxysomal operon. It is suggested, that CsoS1D may moduly function of CBs and influences the shell permeability. Furthermore it could be showed, that CsoS1D seems to reduce the number of CBs with shell defects. (Bonacci et al., 2011) CsoS2 is a further shell protein. With a size of 80-90 kDa it is a large protein, but the function is still unknow (Batger et al., 2002 (a); Yeates et al., 2008). CsoS2 is characteristic for α-type carboxysomes. It seems to have no enzymatic activity, but the localisation primaliry in the peripheric of the carboxysome could be demonstrated (Yeates et al., 2008). CsoS4A and CsoS4B are responsible for the vertices of the icosahedral shell, concluding this from crystal structures. In the carboxysome both proteins are present in equimolar, but very low amounts. Knockout of both genes gives rise to more leaky carboxysomes, indicating a loss in permeability barrier function of the shell of the carboxysome. (Cai et al., 2009) In Table 1 a summary of the carboxysomal proteins also including CsoS1D is shown.
Table1: Functions of the proteins coded in the carboxysomal operon of Halothiobacillus neapolitanus included CsoS1D.
| Protein | Function |
|---|---|
| CsoS1A | Main shell protein, building up the structure of the carboxysome. |
| CsoS1B | Main shell protein, building up the structure of the carboxysome. |
| CsoS1C | Main shell protein, building up the structure of the carboxysome. |
| CsoS1D | Shell protein. It may moduly function of CBs and influences the shell permeability. |
| CsoS2 | Unknown |
| CsoS3 | Carbonic anhydrase (CA). The CA catalyzes the conversion of hydrogencarbonate to gaseous carbon dioxide. |
| CsoS4A | CsoS4A is responsible for the vertices of the icosahedral shell structure. It is indicatet, that this protein plays a role in shell permeability. |
| CsoS4B | CsoS4A is responsible for the vertices of the icosahedral shell structure. It is indicatet, that this protein plays a role in shell permeability. |
References
-
Batger, Price 2002 (a). CO2 concentrating mechanisms in cyanobacteria: molecular components, their diversity and evolution Journal of Experimental Botany, vol. 54, pp. 609-622
-
Batger et al., 2002 (b). Evolution and diversity of CO2 concentrating mechanisms in cyanobacteria Functional Plant Biology, vol. 29, pp. 161-173
-
Bonacci et al., 2011. Modularity of carbon-fixing protein organelle. PNAS, vol. 109, pp. 478-483
-
Cai et al., 2009. The Pentameric Vertex Proteins Are Necessary for the Icosahedral Carboxysome Shell to Function as a CO2 Leakage Barrier PLoS ONE, vol.4, pp. 1-9
-
Cannon, Shively 1983. Characterisation of a Homogenous Preparation of Carboxysomes from Thiobacillus neapolitanus. Archive of Microbiology, vol. 134, pp. 52-59
-
Holthuijzen et al., 1986. Protein composition of the carboxysomes of Thiobacillus neapolitanus. Archive of Microbiology, vol. 144, pp. 398-404
-
Kerfeld et al., 2005. Protein structures Forming the Shell of Primitative Bacterial Organelles. Science, vol. 309, pp. 936-938
-
Reinhold et al., 1990. A model for inorganic carbon fluxes and photosynthesis in cyanobacterial carboxysomes. Canadian Journal of Botany, vol. 69, pp. 984-988
-
So et al., 2004. A Novel Evolutionary Lineage of Carbonic Anhydrase (ε Class) Is a Component of the Carboxysome Shell. Journal of Bacteriology, vol. 186, pp. 623-630
-
Yeates et al., 2011. Protein-based organelles in bacteria: carboxysomes and related microcompartments. Nature Reviews Microbiology, vol. 6, pp. 681-691
 "
"
