Team:Bielefeld-CeBiTec/Project/CO2-fixation
From 2014.igem.org
| Line 72: | Line 72: | ||
<i>Chlorobium thiosulfatophilum</i> is the first organism where this cycle could be observed by Evans, Buchanan and Arnon 1966 (Arnon-Buchanan Cycle).<br> | <i>Chlorobium thiosulfatophilum</i> is the first organism where this cycle could be observed by Evans, Buchanan and Arnon 1966 (Arnon-Buchanan Cycle).<br> | ||
We decided not to work with this cycle because by using it we had to use anaerobic cultivation conditions which we try to avoid.</p> | We decided not to work with this cycle because by using it we had to use anaerobic cultivation conditions which we try to avoid.</p> | ||
| + | |||
| + | <center> | ||
| + | <div class="element" style="margin:10px; padding:10px; text-align:center; width:450px"> | ||
| + | <a href="https://static.igem.org/mediawiki/2014/f/fe/Bielefeld-CeBiTec_2014-10-11_Reductive_tca_cycle.jpg" target="_blank"><img src="https://static.igem.org/mediawiki/2014/f/fe/Bielefeld-CeBiTec_2014-10-11_Reductive_tca_cycle.jpg" width="450px"></a><br> | ||
| + | <font size="1" style="text-align:center;">The reductive citric acid cycle (Arnon-Buchanan). 1. ATP-citrate lyase, 2. malate dehydrogenase, 3. fumarate hydratase, 4. fumarate reductase, 5. succinyl-CoA synthetase, 6. ferredoxin dependent 2-oxoglutarate synthase, 7. isocitrate dehydrogenase, 8. aconitate hydratase, 9. ferredoxin dependent pyruvate synthase, 10. phosphoenolpyruvate synthase, 11. phosphoenolpyruvate carboxylase</font> | ||
| + | </div> | ||
| + | </center> | ||
| + | |||
| + | |||
</div> | </div> | ||
</div> | </div> | ||
| Line 92: | Line 101: | ||
The pathway has been found in <i>Clostridium thermoacetium</i> which is a strictly anaerobic bacteria (acetogens).<br> | The pathway has been found in <i>Clostridium thermoacetium</i> which is a strictly anaerobic bacteria (acetogens).<br> | ||
Because this pathway also occurs only under anaerobic conditions we decided to not use it for our project.</p> | Because this pathway also occurs only under anaerobic conditions we decided to not use it for our project.</p> | ||
| + | <center> | ||
| + | <div class="element" style="margin:10px; padding:10px; text-align:center; width:650px"> | ||
| + | <a href="https://static.igem.org/mediawiki/2014/7/74/Bielefeld-CeBiTec_2014-10-12_Reductive_acetyl_coa_cycle.jpg" target="_blank"><img src="https://static.igem.org/mediawiki/2014/7/74/Bielefeld-CeBiTec_2014-10-12_Reductive_acetyl_coa_cycle.jpg" width="650px"></a><br> | ||
| + | <font size="1" style="text-align:center;">The reductive acetyl coA (Wood-Ljungdahl) pathway. 1. formate dehydrogenase, 2. formyl-tetrahydroforlate synthetase, 3. formyl-methanofuran dehydrogenase, 4. formyl-methanofuran:tetrahydromethanopterin formyltransferase, 5. methenyl-tetrahydroforlate cyclohydrolase, 6. methenyl-tetrahydromethanopterin cyclohydrolase, 7. methylene-tetrahydroforlate dehydrogenase, 8. methylene-tetrahydromethanopterin dehydrogenase, 9. methylen-tetrahydroforlate reductase, 10. methylen-tetrahydromethanopterin reductase, 11. CO, dehydrogenase / acetyl-CoA synthase</font> | ||
| + | </div> | ||
| + | </center> | ||
</div> | </div> | ||
</div> | </div> | ||
| Line 112: | Line 127: | ||
The origin of this cycle is Chloroflexus aurantiacus.<br> | The origin of this cycle is Chloroflexus aurantiacus.<br> | ||
We decided to did some research about this cycle but our main focus lies on the Calvin cycle.</p> | We decided to did some research about this cycle but our main focus lies on the Calvin cycle.</p> | ||
| + | <center> | ||
| + | <div class="element" style="margin:10px; padding:10px; text-align:center; width:550px"> | ||
| + | <a href="https://static.igem.org/mediawiki/2014/c/cd/Bielefeld-CeBiTec_2014-10-12_3hydroxypropionate_cycle.jpg" target="_blank"><img src="https://static.igem.org/mediawiki/2014/c/cd/Bielefeld-CeBiTec_2014-10-12_3hydroxypropionate_cycle.jpg" width="550px"></a><br> | ||
| + | <font size="1" style="text-align:center;">The 3-hydroxypropionate (Fuchs-Holo) bicycle. 1. acetyl-CoA carboxylase, 2. malonyl-CoA reductase, 3. propionyl-CoA synthase, 4. propionyl-CoA carboxylase, 5. methylmalonyl-CoA epimerase, 6. methylmalonyl-CoA mutase, 7. succinyl-CoA:(S)-malate-CoA transferase, 8. succinate dehydrogenase, 9. fumarate hydratase, 10. (a,b,c) trifunctional (S)-malonyl-CoA a/(β)-methylmalonyl-CoA (b)/(S)-citramalyl-CoA lyase, 11. mesaconyl-C1-CoA hydratase, 12. mesaconyl-CoA C1-C4 CoA transferase, 13. mesaconyl-C4-CoA hydratase</font> | ||
| + | </div> | ||
| + | </center> | ||
</div> | </div> | ||
</div> | </div> | ||
| Line 130: | Line 151: | ||
<div class="content"> | <div class="content"> | ||
<p>The Calvin cycle is the light independent reaction of the photosynthesis. Photosynthesis is done by all plants and some sulfurbacteria. The products of the photosynthesis ATP and NADPH are used for the Calvin cycle. By using ATP and NADPH carbohydrates were produced. In the cycle carbon dioxide is taken up and higher sugars were produced.</p> | <p>The Calvin cycle is the light independent reaction of the photosynthesis. Photosynthesis is done by all plants and some sulfurbacteria. The products of the photosynthesis ATP and NADPH are used for the Calvin cycle. By using ATP and NADPH carbohydrates were produced. In the cycle carbon dioxide is taken up and higher sugars were produced.</p> | ||
| + | <center> | ||
| + | <div class="element" style="margin:10px; padding:10px; text-align:center; width:550px"> | ||
| + | <a href="https://static.igem.org/mediawiki/2014/c/cd/Bielefeld-CeBiTec_2014-10-12_3hydroxypropionate_cycle.jpg" target="_blank"><img src="https://static.igem.org/mediawiki/2014/c/cd/Bielefeld-CeBiTec_2014-10-12_3hydroxypropionate_cycle.jpg" width="550px"></a><br> | ||
| + | <font size="1" style="text-align:center;">The reductive pentose phosphate (Calvin-Benson-Bassham) cycle. 1. </font> | ||
| + | </div> | ||
| + | </center> | ||
</div> | </div> | ||
</div> | </div> | ||
Revision as of 18:23, 12 October 2014
CO2 fixation
Short summary
In the second module we aim to use a carboxysome which is found in cyanobacteria or purple sulfurbacteria. With this compartment we want to create a Calvin-Benson cycle in E. coli. In addition we would like to compare the efficiency of the carboxysome with a free RuBisCO (Ribulose-1,5-bisphosphate-carboxylase-oxygenase), the 3-Hydroxypropionate cycle or other types of carboxysomes. The product of the fixation will be pyruvate which can be used for production of different metabolites like for example Isobutanol.
Here you will find the results of the CO2 fixation.

Carbon dioxide
Increasing amounts of carbon dioxide has become a major problem in this century. Because of the industrialization typical handmade ware is built by machines which produces carbon dioxide. By changing nearly every production site to industrial production the amount of emission has increased a lot. An additional factor is industrial livestock farming by which methane and carbon dioxide is produced.
The typical balance between consumption and production of carbon dioxide is harmed. The number of forests decreases and the amount of emission increases year by year. Because of this many specialists work on a method to fight the excess of carbon dioxide in the atmosphere.
With our project we also want to engage this problem by using carbon dioxide as a carbon source for organic products.
Kinds of carbon dioxide fixation
The citric acid cycle [TCA cycle] (oxidative) is one of the main cycles used by all aerobic organisms. It is used to generate energy through oxidation of acetate which is derived from different substances like fats, carbohydrates and proteins over carbon dioxide and ATP. The reductive citric acid cycle runs in reverse. That means it uses carbon dioxide and ATP to generate carbohydrates, fats and proteins.
Chlorobium thiosulfatophilum is the first organism where this cycle could be observed by Evans, Buchanan and Arnon 1966 (Arnon-Buchanan Cycle).
We decided not to work with this cycle because by using it we had to use anaerobic cultivation conditions which we try to avoid.

The reductive citric acid cycle (Arnon-Buchanan). 1. ATP-citrate lyase, 2. malate dehydrogenase, 3. fumarate hydratase, 4. fumarate reductase, 5. succinyl-CoA synthetase, 6. ferredoxin dependent 2-oxoglutarate synthase, 7. isocitrate dehydrogenase, 8. aconitate hydratase, 9. ferredoxin dependent pyruvate synthase, 10. phosphoenolpyruvate synthase, 11. phosphoenolpyruvate carboxylase
The reductice acetyl CoA pathway also called Wood-Ljungdahl pathway (1965) uses carbon dioxide as electron acceptor and hydrogen as electron donor for biosynthesis. The product of this cycle is acetyl CoA which is used for several biological reactions.
The pathway has been found in Clostridium thermoacetium which is a strictly anaerobic bacteria (acetogens).
Because this pathway also occurs only under anaerobic conditions we decided to not use it for our project.

The reductive acetyl coA (Wood-Ljungdahl) pathway. 1. formate dehydrogenase, 2. formyl-tetrahydroforlate synthetase, 3. formyl-methanofuran dehydrogenase, 4. formyl-methanofuran:tetrahydromethanopterin formyltransferase, 5. methenyl-tetrahydroforlate cyclohydrolase, 6. methenyl-tetrahydromethanopterin cyclohydrolase, 7. methylene-tetrahydroforlate dehydrogenase, 8. methylene-tetrahydromethanopterin dehydrogenase, 9. methylen-tetrahydroforlate reductase, 10. methylen-tetrahydromethanopterin reductase, 11. CO, dehydrogenase / acetyl-CoA synthase
The 3-hydroxypropionate bicycle produces 3-hydroxypropionate by consuming carbon dioxide. The enzymes of this cycle are not especially oxygen sensitive.
The origin of this cycle is Chloroflexus aurantiacus.
We decided to did some research about this cycle but our main focus lies on the Calvin cycle.

The 3-hydroxypropionate (Fuchs-Holo) bicycle. 1. acetyl-CoA carboxylase, 2. malonyl-CoA reductase, 3. propionyl-CoA synthase, 4. propionyl-CoA carboxylase, 5. methylmalonyl-CoA epimerase, 6. methylmalonyl-CoA mutase, 7. succinyl-CoA:(S)-malate-CoA transferase, 8. succinate dehydrogenase, 9. fumarate hydratase, 10. (a,b,c) trifunctional (S)-malonyl-CoA a/(β)-methylmalonyl-CoA (b)/(S)-citramalyl-CoA lyase, 11. mesaconyl-C1-CoA hydratase, 12. mesaconyl-CoA C1-C4 CoA transferase, 13. mesaconyl-C4-CoA hydratase
The Calvin cycle is the light independent reaction of the photosynthesis. Photosynthesis is done by all plants and some sulfurbacteria. The products of the photosynthesis ATP and NADPH are used for the Calvin cycle. By using ATP and NADPH carbohydrates were produced. In the cycle carbon dioxide is taken up and higher sugars were produced.
Calvin cycle
The Ribulose 1,5-bisphosphate carboxylase oxygenase is the most abundant enzyme of the world because it occurs in every plant in a high concentration. The reaction of this enzyme is essential for the functionality of the Calvin cycle because it uses the atmospheric carbon dioxide to generate two tricarbohydrates out of one pentacarbohydrate. The problem of the RuBisCO is that is also accepts oxygen with a higher percentage as cofactor. The following reaction results in one dicarbohydrate and one tricarbohydrate.
The RuBisCO consists of two subunits, a small and a large subunit. In higher plants the RuBisCO is formed out of four large and four small subunits. In smaller organisms the RuBisCO is only formed out of two proteins each.
We aim to use the RuBisCO from two different organisms which are mentioned below.
Andersson, 2008
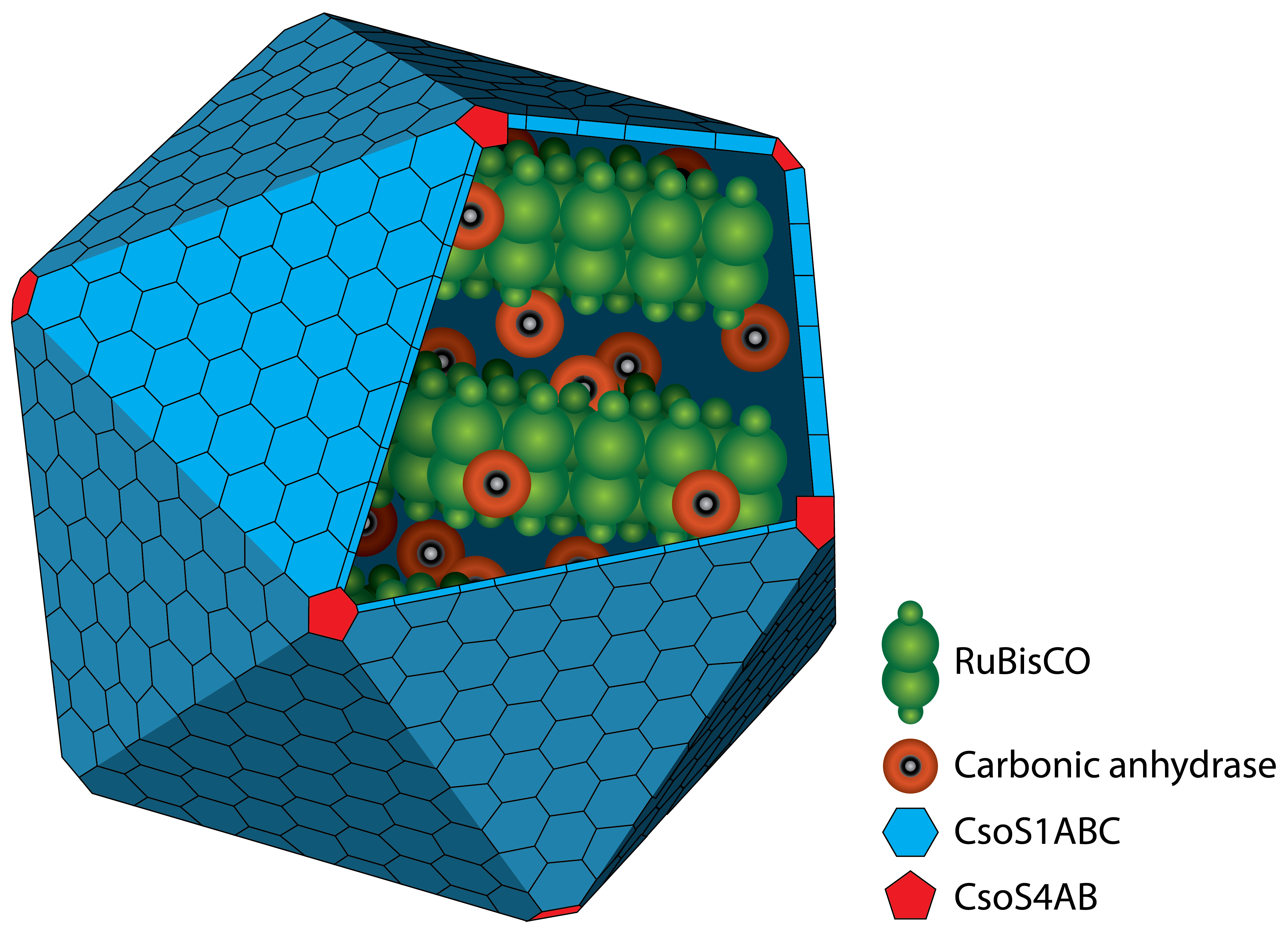
Carboxysom
A carboxysom is an intracellular microcompartiment with a protein shell. The protein shell consists of two different types of proteins. Pentamers are used for the vertices of the icosaeder and hexamers for the facets. In the interior there are two different enzymes. On the one hand there is the RuBisCO which catalyses the reaction like described above. On the other hand there is the carbonic anhydrase which converts hydrogen carbonat (HCO3+) to carbon dioxide. The resulting carbon dioxide is the substrate for the RuBisCO.
The advantage of the microcompartiment is that the concentration of carbon dioxide inside can be much higher than outside which increases the efficiency of the RuBisCO.
There are two different types of carboxysomes which are classified by the habitat of the organism. It is found in all cyanobacteria and some chemolitoautotrophic bacteria. A deletion mutant for a single gene of the cluster results in a conditionally lethal phenotype which requires high concentrations of carbon dioxide.
Halothiobacillus neapolitanus
This organism is a gram negative proteobacteria which is classified as a purple sulfur bacteria. It is obligate aerob. It is know to tolerate and metabolize high amounts of sulfide concentrations.
Halothiobacillsu neapolitanus is a chemolitoautotroph modell organism for the carboxysome with a diameter of 120nm. This type of carboxysome dominates in oligotrophic oceans (cso-carboxysome or alpha type). It has to be distinguished from the ccm-carboxysome found in several other marine- and freshwater cyanobacteria (beta type).
The first occurence was 1957 by Parker et al.
Synechoccus elongatus
Synechococcus elongatus is a cyanobacteria which is found in surface waters and freshwater. It carries a carboxysome like Halothiobacillus neapolitanus but it has the beta type.
Genetical approach
Our goal is to bind carbon dioxide for which we searched for several pathways. We decided to work with the calvin cycle because there are only three enzymes missing to enable the whole cycle. The possibility, the 3-Hydroxypropionate bicycle, would be also possible for our project but there are too many enzymes missing.
The first missing enzyme is the Sedoheptulose 1,7-bisphosphatase. It was successfully transformed by Stolzenberger et al. In 2013. The origin of this enzyme is Bacillus methanolicus. We also aim to introduce this enzyme. For the RuBisCO we decided to use the carboxysome of Halothiobacillus neapolitanus which was successfully transformed by Bonacchi et al. In 2011. The phosphoribulokinase is taken from Bacillus subtilis which was functionally tested before by Parikh et al. in 2006.
The gene cluster of the carboxysome carries many illegal restriction sites in some sequence parts. Because of this we decided to synthesize some parts of the sequence which we will assemble with the original sequence. By synthesizing the sequence we are able to optimize the codon usage for E. coli.
In addition we want to compare the RuBisCO of H. neapolitanus with the RuBisCO of Synechococcus elongatus. By this comparison we want to identify the optimal enzyme for carbon dioxide fixation in E. coli.
If it is possible to enable the whole cycle E. coli should be able to grow with electricity and carbon dioxide. We think of feeding a pentacarbohydrate to feed the Calvin cycle if the efficiency is not high enough.
References
-
Andersson, 2008. Catalysis and regulation in Rubisco. Journal of Experimental Botany, vol. 59, pp. 1555-1568
 "
"