Team:ETH Zurich/blog
From 2014.igem.org
| Line 5: | Line 5: | ||
<html><br/> <br/> </html> | <html><br/> <br/> </html> | ||
<!-- ADD YOUR ARTICLE HERE --> | <!-- ADD YOUR ARTICLE HERE --> | ||
| - | <html><div id=' | + | <html><div id='Container'></html> |
<div class="filter" data-filter="all">Show All</div> | <div class="filter" data-filter="all">Show All</div> | ||
<div class="filter" data-filter=".category-1">Category 1</div> | <div class="filter" data-filter=".category-1">Category 1</div> | ||
Revision as of 14:17, 11 October 2014
Blog
We have uploaded our chronological progress in several articles which are classified by subject. Click on the mosaic to choose the articles you want to read. If you prefer a weekly progress of our project, go to "Weekly updates".
Week 16 : choosing an AHL pair, improving diffusion
Monday 8th September
- We have enough crosstalk results to decide which pair of quorum sensing systems we will use : Las and Lux. See this article for more details.
- Diffusion experiment (trying to have a correlation between channel length and time of fluorescence, having cells at the end of a channel and adding pure HSL at the other end of the channel, see this article for more details on the setup) : We tried lower concentrations of pure HSL and the wells start shining at different moments, but we still don't have any correlation between channel length and starting time of fluorescence, because our chip seems to be leaky and some wells dried completely. However, this experiment enables us to check that fluorescence appears after a reasonable time and that channel length probably doesn't influence this time too much.
- On the modeling side, we are not able to retrieve diffusion coefficient of HSL from this diffusion experiment but we can use a parameter from literature.
Discussions with experts
Tuesday, September 9th
Complexity in town planning and complexity in religion
In the last days Stefanie met an architect and a priest to discuss complexity in their occupational field. Both conversations were highly interesting and at the same very different. It was fascinating and inspiring to once change the point of view and to approach complexity from new positions. For the priest, Josef Fuisz, the struggle of men with complexity might be represented by the following psalm (42.5):
Why, my soul, are you downcast?
Why so disturbed within me?
Put your hope in God,
for I will yet praise him,
my Savior and my God.
Other interesting interviews were done in various fields. For example a philosophy teacher, a physicist, a nano-technologist and a theoretical computer scientist were partners for talks. From these interviews we expect to gain insight into the diversity of complexity. It became even more clear than before that complexity can be found in many different fields.
Visit our interview page for the details of each conversation.
Week 15 : Integrase parameters retrieved from Bonnet's paper, problems with integrase experiments
Monday 1st September
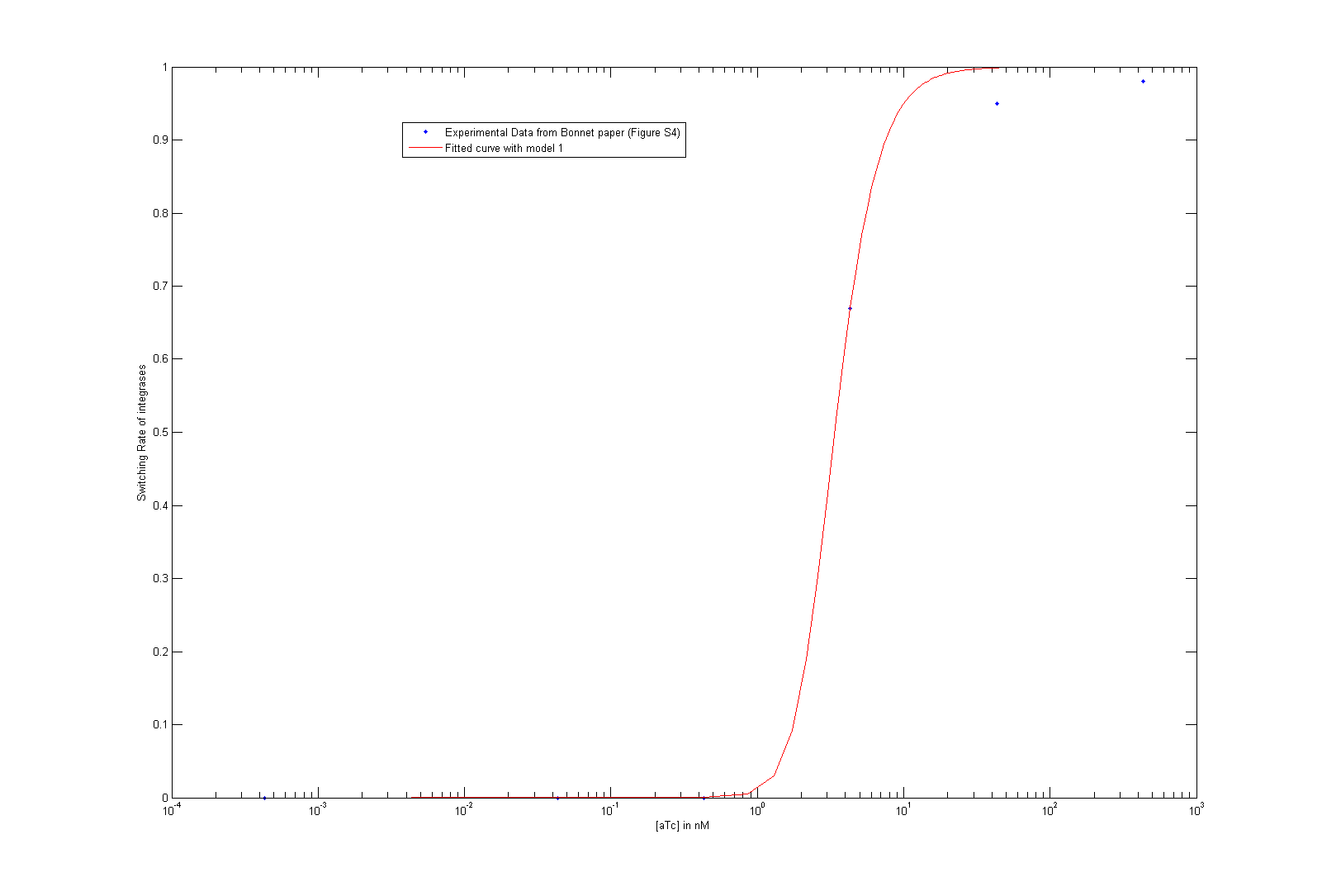
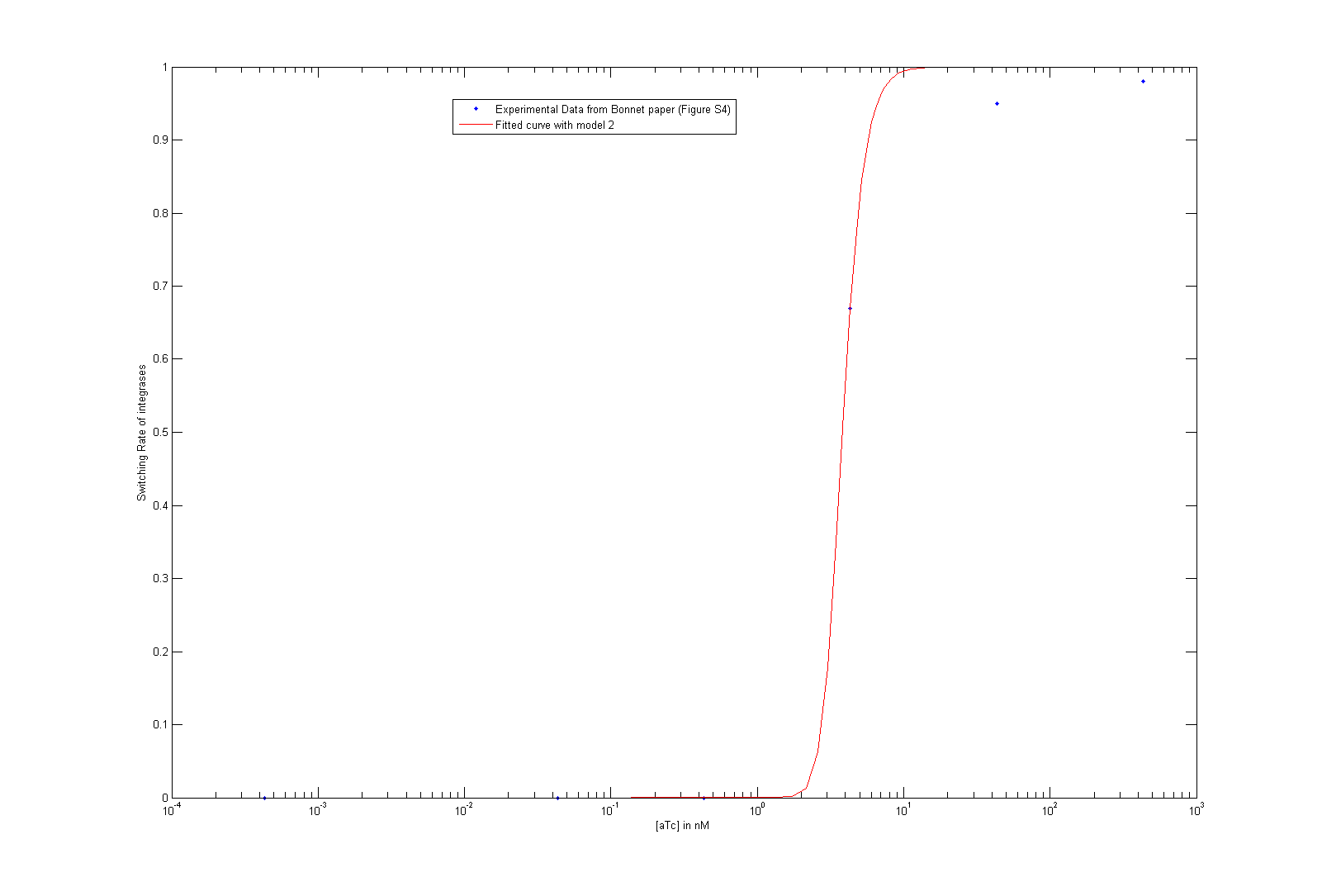
- We finally managed to retrieve integrase parameters from Bonnet et al.'s [9] paper steady state behavior curve. You can check the corresponding article for more details. We can get Hill function parameters this way, but still need dynamic data from our own experiments to retrieve dynamic parameters (i. e. degradation rates).
- Integrases don't seem to switch the terminator at the moment, maybe because of a mutation that we found in their sequence.
Integrase parameters
Friday, August 29th
In order to predict accurately the experiments we designed, we defined two different strategies to retrieve parameters for the integrase system.
As we did not find these parameters on the web, we used the data given by Bonnet et al.'s paper "Amplifying genetic logic gates" [9] . The figure S4 is particularly interesting, even if it does not give any information on the dynamics of the system. We modeled and wrote the equations corresponding to Bonnet's experiment.
At first, we try to fit the dynamic parameters of the system with only the steady states values. To fit the parameters with the data, we first try to use a minimum search for the difference between the data and the parametric function. Then, using these first approximations, we tried to do a Markov Chain Monte Carlo approach to find a more suited curve. As these two methods are strongly prior dependent, we planned another step of screening in order to have meaningful results. However, our knowledge on the parameters, the number of data points compared to the high number of parameters did not give any meaningful results.
We then decided to simplify our system :
- We consider that Bxb1a and Bxb1b have the same behavior. Therefore, they don't appear as differentiated in our system anymore.
- The paper uses the tet system to induce their system. This was modeled explicitly before. We approximated this part to a Hill function, which takes leakiness into consideration. The parameters for the Hill function are the result from a study of an other iGEM team UCSF from last year. Please visit their page for more information. The same biobrick is indeed used in their work and in Bonnet's paper.
- We consider that the dimer of the integrase and the binding to DNA were at quasi steady-state.
As we tried to fit the steady state, we obtained two different models, depending on which assumption we made. As these two models fit the Bonnet's curve well, we used them both to derive more precisely the dissociation constant of the dimerization reaction and the one of the DNA binding reaction. Each of them appear in one of the lumped parameters we fitted.
By considering reasonable order of magnitude for other parameters present in the lumped parameters, we find that the value of the dissociation constants are both of the order of 10-4. This order of magnitude is not surprising at all, as we know that the binding of integrases to DNA is very specific (which is underlined by the low value of the dissociation constant of this reaction) and that integrases are more stable as dimer than as monomer.
Diffusion Experiments
Monday, 25th August
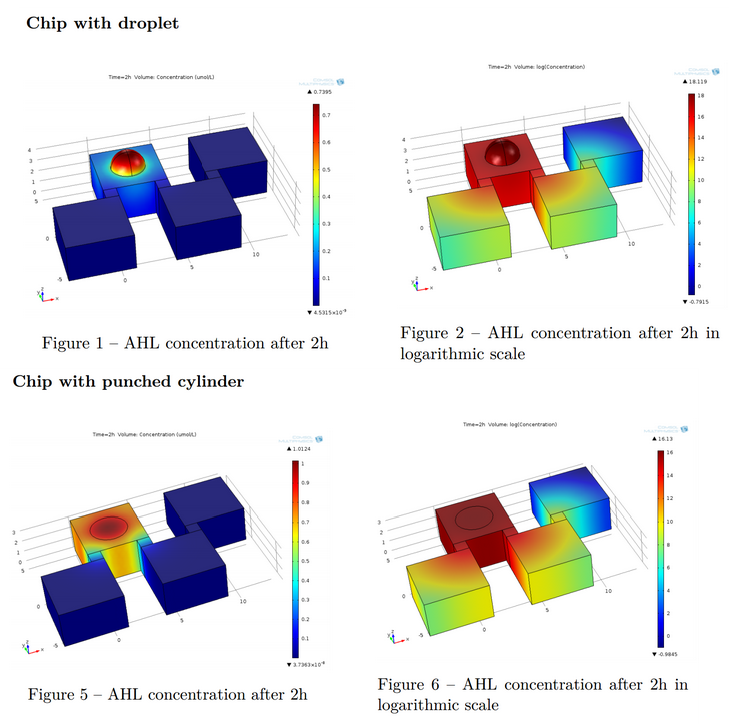
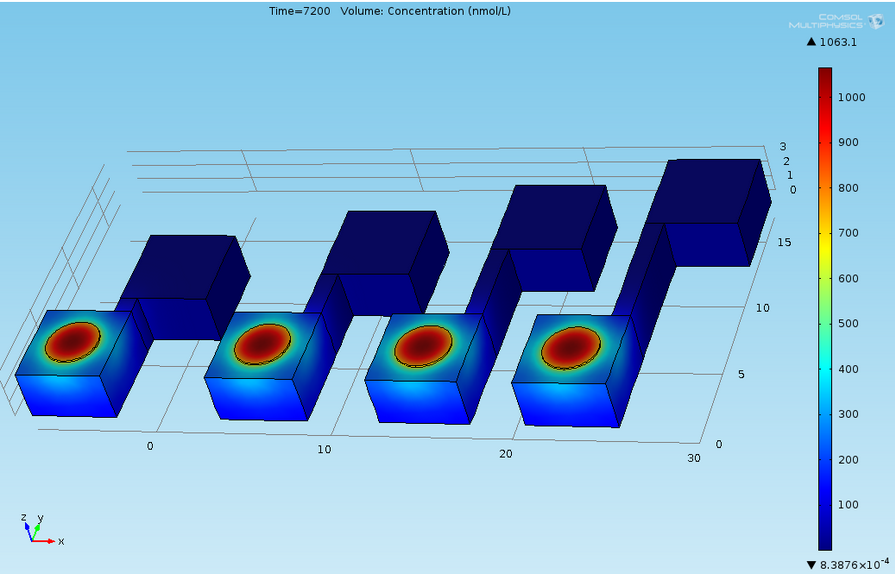
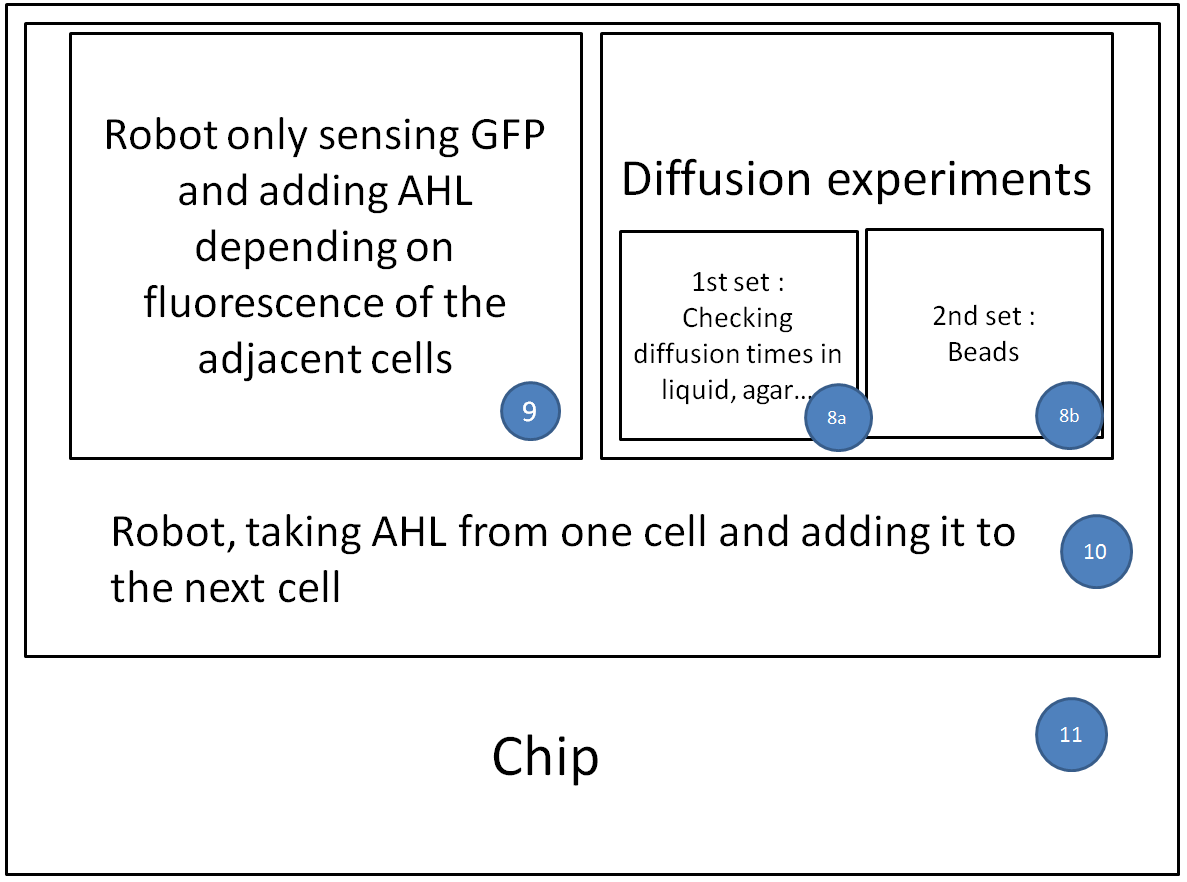
Now that we tested the different constructs for leakiness, dose response and cross talk, we are investigating in the diffusion rate of AHL. Therefore we designed a chip with chambers connected by channels of different length (see figure). In one chamber we added a strain containing a regulator and a sensor plasmid, to the second chamber we added AHL or an AHL producer strain. As soon as AHL is diffused to the former chamber it induces sfGFP production on the sensor plasmid. The time it takes until we can measure the sfGFP signal and the length of the channel gives us information about the velocity of AHL diffusion.
Three main experiment designs were tested and modified: the agar-design, the liquid-design and the beads-design.
- For the agar-design we filled the chamber and channels with LB-agar or in a second attempt with agarose only. Subsequently, we punched a cylindric hole in each chamber. The capacities were filled with regulator/sensor strain culture or AHL/AHL producer strain culture, respectively. After 3 to 4 h at 37 °C we could observe an increase in the sfGFP signal. Since we used comparably high concentration of AHL in our first attempts, we could not detect a delayed sfGFP signal for chambers connected by a longer channel. The experiment will be repeated using lower concentrations. However, conducting the experiment using the agar-design, we encountered a problem: after some hours the LB-agar/agarose started to shrink and dried out. Thus, the agar-design cannot be used without modifications for experiments of longer duration.
- In an attempt we only left a small agar fragment in each channel and filled the rest with liquid medium, so as to decrease chance of it drying out, while avoiding at the same time that bacteria from one chamber reach the other chamber.
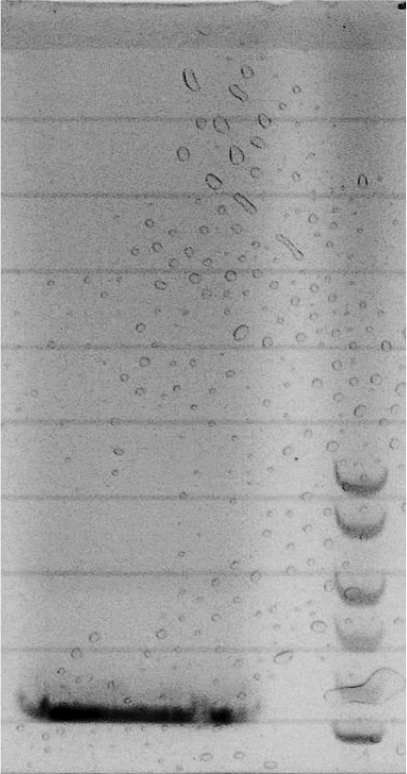
- The bead-design consists of an alginate bead for each chamber and liquid medium in between. The beads either contained regulator/sensor-bacteria or AHL.

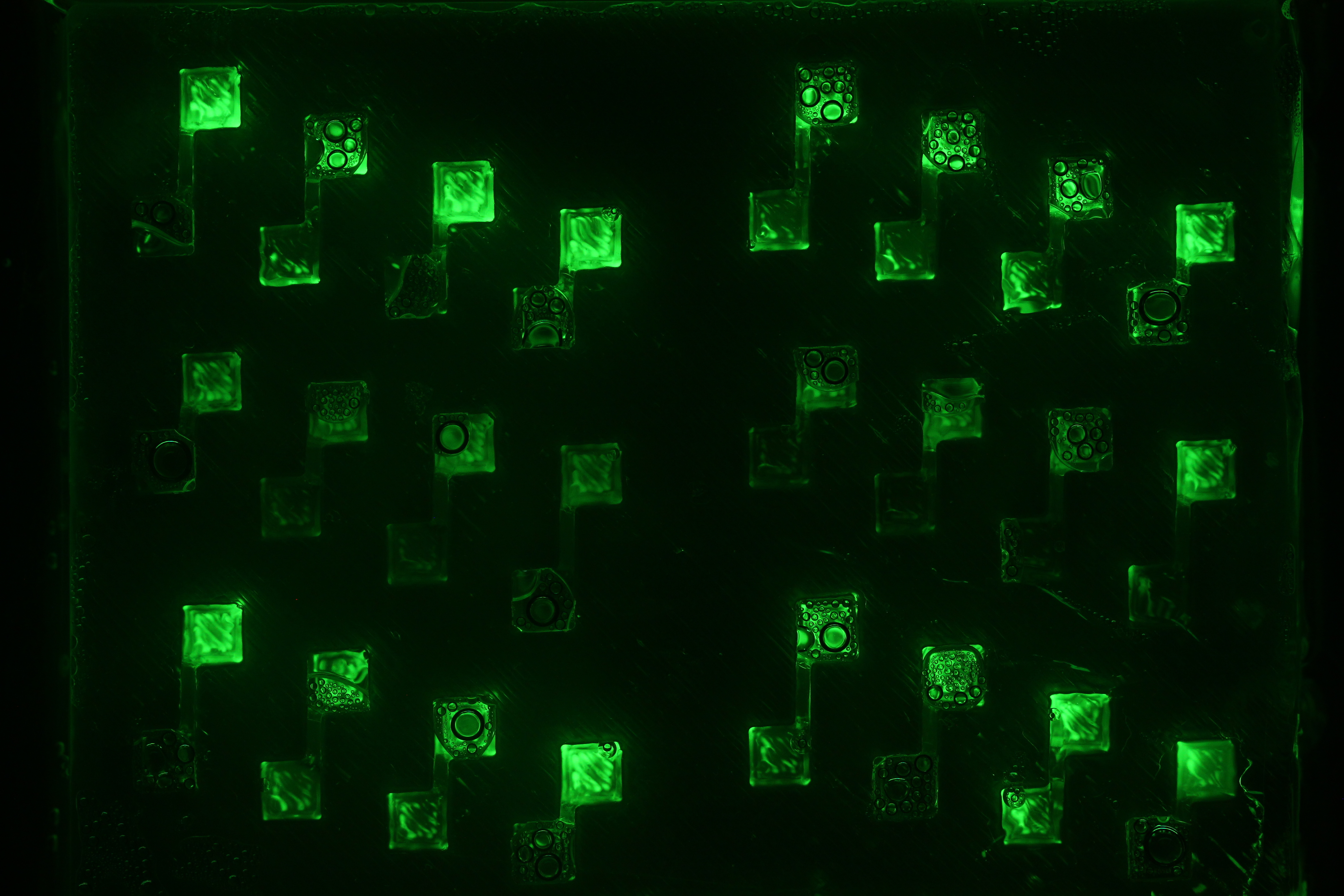
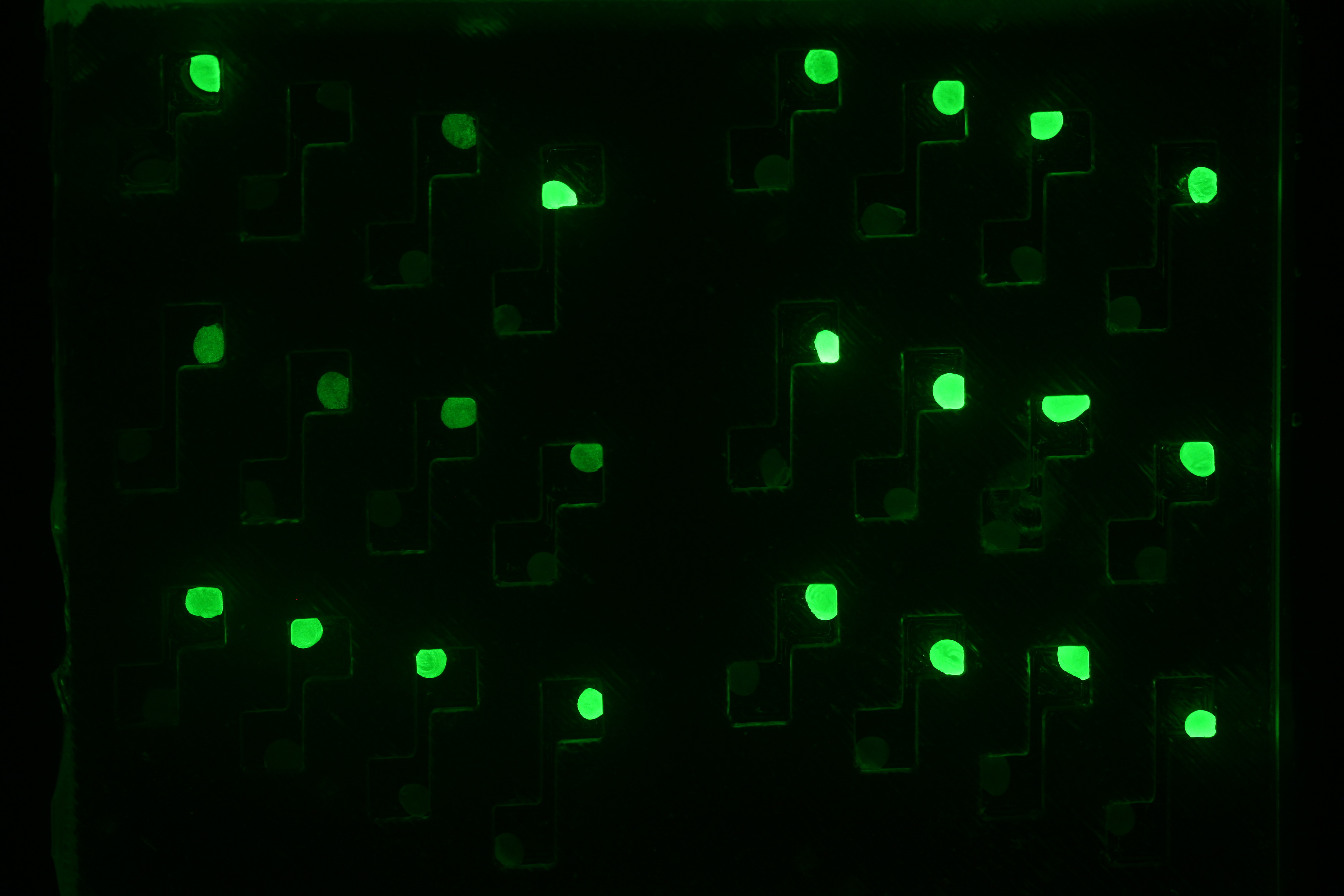
Week 14 : Diffusion experiments started, simulated with Comsol, and fitting of quorum sensing dynamics
Monday, August 25th
- We carried diffusion experiment with different designs : punched PDMS, liquid, beads. For every design we added high concentrations of pure AHL in the sender well, so cells are glowing a lot. However because of this high concentration, diffusion is very high and at a given AHL concentration, receiving cells are glowing at the same time for all 4 channel lengths. Check the corresponding article for more details.
- We have a Comsol simulation for these experiments that explains why at this concentration of AHL (1mM), all 4 wells glow at the same time irrespectively of channel length. Check this article for more details.
- We could fit degradation rates of external, internal AHL and GFP thanks to quorum sensing dynamic curves.
Check the corresponding article for more details.
Week 13 : Steady state quorum sensing fitted and human practice survey launched !
Wednesday, August 20th
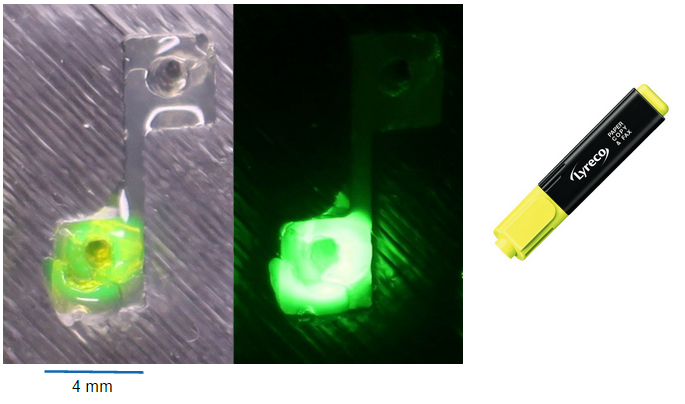
- Our first diffusion try with highlighter ink !
The design of punched agar doesn't seem to be the best but at least we can see something. Diffusion experiments with different designs are running now.
- We have been fitting quorum sensing experiments at steady state to a Hill function and found some quorum sensing parameters.
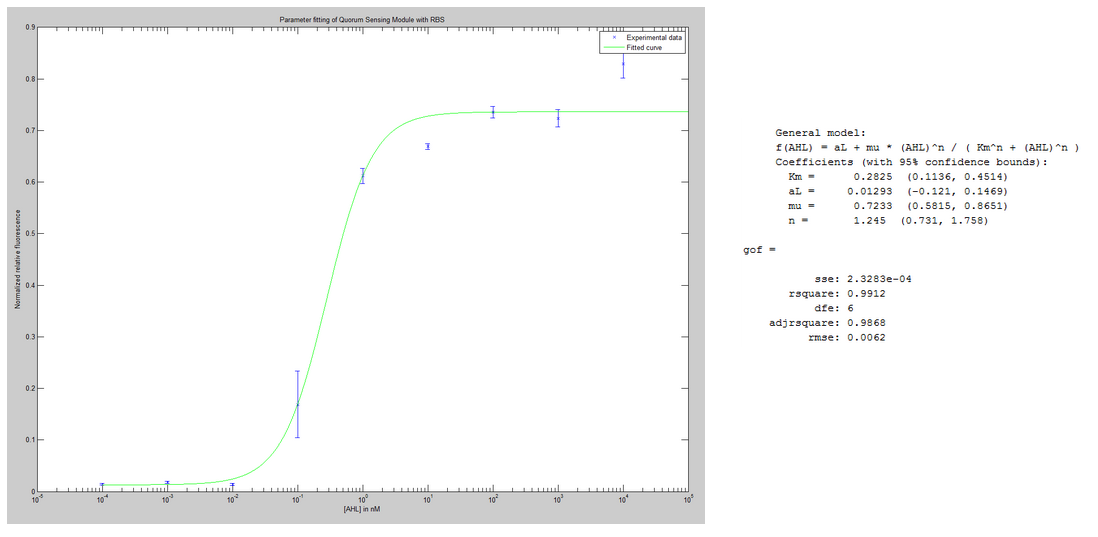
- We wrote a survey in English, in French and in German on complexity for our human practice project. We expect 500 responses.
Fitting quorum sensing Hill functions parameters
Wednesday, August 18th
The plasmids for quorum sensing experiments concerning the Lux system are made and operational to be used for experiments. We have three different plasmids:
- Lux promoter folowed by GFP gene
- Lux promoter followed by cisRNA and then by GFP
- Lux promoter controlling the expression of taRNA and cisRNA. The cisRNA coding gene is followed by GFP.
The third plasmid corresponds to a riboregulating system. The second plasmid allowed us to validate the crucial role of taRNA in this riboregulating system.
On the modeling point of view, we only tried to fit the data coming from experiments done with plasmid one (with no riboregulating system) and plasmid three (with an effective riboregulating system). They were the only one to show an answer to a change of input that was not on the noise level.
The experimental setting was to inject different concentration of the signaling molecule AHL at the same time point into different bacterial colonies. The factor that we want to observe is the strength of the activation of the Lux promoter. The read-out is made by the fluorescence. The data was gathered over several time points in order to be able to observe the dynamic behavior. More precisely, the read-out of the promoter activation is made by the relative fluorescence, which corresponds to the ratio of the absolute fluorescence over the Optical Density (OD). We consider that the concentration in GFP per cell is proportional to the relative fluorescence. As we try to avoid fitting the saturation events, we chose to normalize with respect to the highest value before a certain amount of time, which corresponds for each experiment to the apparition of the saturation.
As a first approach, we only try to fit the steady state value. As the signaling molecule is inducing the production of GFP, we chose to use an Hill function to fit the function [GFP] = f([AHL]) at steady state.
If Hill functions fit these two curves good, the parameters used are surprising. For our system, we suppose that AHL binds to the regulator protein LuxR. This complex called Rlux dimerizes before binding to the promoter to induce its expression. Thus, the dimerization event makes cooperativity emerge from AHL molecules. It is translated for a Hill function by n > 1. In this particular case, one could expect to find n=2 (as it is a dimerization event). Nevertheless, the n found are on the order of magnitude of 1 (0.89 for the non-riboregulated promoter plasmid and 1.22 for the ribo-regulated promoter plasmid). The data points were collected in triplicates and this allow us to have a look at the variability (namely standard deviation) around each value. Where the switch happens, the variability is higher (it is expected because the output GFP is more sensitive to variation of the input [AHL] around the switching point). Therefore, the inflexion points could need some improvment, by taking more points around this identified sensitive region. Moreover, this critical concentration of AHL was the one that was expected given the litterature and our system. We observe that it needs a higher [AHL] for the riboregulated system to be activated than for the non-riboregulated system.
We modeled leakiness as an offset in the Hill function : [GFP] = g([AHL]) = f([AHL]) + aLeakiness. We obtain the expected information that the signal over noise ratio is greater by one order of magnitude for the riboregulated system than for the non-riboregulated system. A drawback from the riboregulated system is that the amplitude of the signal corresponds to the amplitude of the noise in the non-riboregulated system.
The experimental design also allowed us to detect eventual cross-talk with other signaling molecules, the one corresponding to the las system and the one corresponding to the rhl system. No cross-talk was observed with the signaling molecule of the rhl system. However, the las system signaling molecule activated the Lux promoter. We chose to model this cross-talk, which corresponds to the induction of a promoter, by an Hill function. This fitting allowed us to verify that the Hill function was adapted to describe a cross-talk phenomena. The derived parameters indicated that the concentration in the las signaling molecule had to be high in order to have an effect on our system (It was two orders of magnitude away from the needed concentration of AHL to activate the system). Nevertheless, once activated, the effects of these crosstalk are not to be neglected. Check the following article for more information on the experimental setting.
These results are promising and we look forward to fit the dynamic data of this system.
Week 12 : Nice cross-talk results, problems with producer strains, and with synthesis of our Las riboregulated sensor
Wednesday, August 13th
- We have a lot of results for cross-talk experiments. Check the corresponding article for more details. For the moment lux promoter doesn't respon to other AHLs. This is a good step towards orthogonality.
- Current challenges :
- AHL producer strains are not producing enough. We have to improve the RBS.
- GenScript has problems to produce our Las sensor with riboregulator RR42.
- We have a project description !
First cross talk experiments
Friday, 11th August
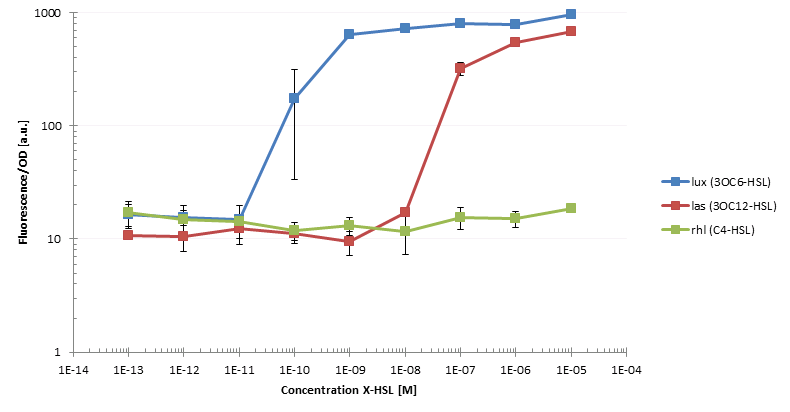
After having constructed the necessary regulator and sensor plasmids we were able to start first cross talk experiments. Therefore we transformed competent cells with different combinations of regulator and sensor plasmids. One example would be the strain siG0024 which contains the regulator plasmid piG0041 producing LuxR under a constitutive promoter and the sensor plasmid piG0051 that encodes for sfGFP. The latter is under control of a pluxR promoter, hence it will be transcribed in the presence of LuxR and Lux. To assess crosstalk and leakiness we conducted multiple experiments: after adding one of the three AHLs to the cultures we analyzed the raise in sfGFP production using a microtiterplate reader. In a first step we measured the dose response of constructs with different ribosomal binding sites (RBSs) and with or without riboregulator. We could observe differences not only in sensitivity, but also in leakiness; some strains expressed GFP even without AHL induction. To give an example: we evaluated the sensitivity of the siG0024 strain to different concentrations of 3OC6-HSL (LuxI product) and compared its characteristics to those of other constructs. Additionally, we measured the sensitivity of siG0024 to 3OC12-HSL (LasI product) and C4-HSL (RhlI product). Indeed, we could observe the induction of sfGFP transcription by comparably low levels of 3OC12-HSL. Crosstalk can be observed between the Las and the Lux system. To simulate a situation most similar to the final experiment, we added the diluted supernatant of AHL producer cells to the test cultures. However, we could not detect any GFP production as reaction to the supernatant. We assume that the AHL concentration is not high enough to induce transcription. By constructing stronger RBSs on the producer plasmids we aim at increasing the AHL production. All these informations we will use to design a system tailored to our plans.
The figure shows the dose response of xxx to 3OC12-HSL, 3OC6-HSL and C4-HSL. Both 3OC12-HSL and 3OC6-HSL induce production of GFP, although at different concentrations. This is an example for cross talk between the lux and the las system.
Week 11 : PDMS chip built, First quorum sensing experiment
Wednesday, August 6th
- We have a first draft of our abstract.
- We have results for our first quorum sensing experiment .
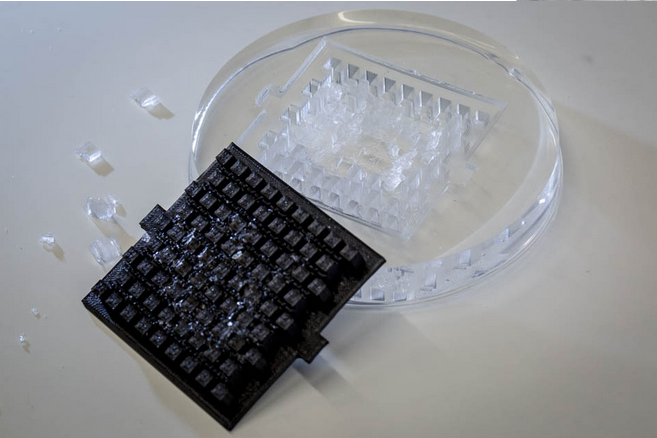
- We have now a proper PDMS chip for diffusion experiments.
- We have a simple GFP production model for simulation of these quorum sensing experiments.
- In the next weeks we will fit quorum sensing parameters thanks to the first quorum sensing experiments and our simple GFP production model, refine this Comsol diffusion model with the right quorum sensing parameters and then fit this diffusion model to diffusion experiments to find the diffusion constant.
Week 10: Chip design, Plasmid assembly, Bead production
Wednesday, July 30th
- We designed two possibilities for the chip: either cells laying on agar or punched cylinders in the agar.
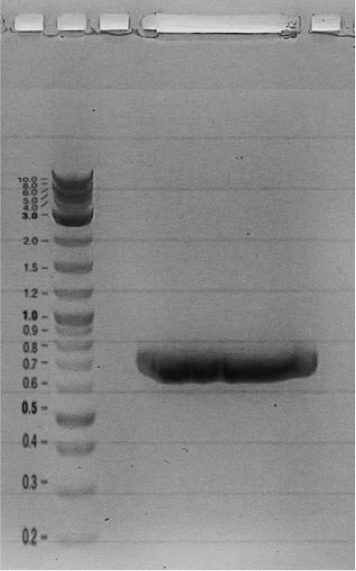
- 8 out of 21 plasmids were positively sequenced. 7 further plasmids are sent for sequencing.
- We will start cross-talk experiments this week. It should help us choose an orthogonal AHL pair among three different quorum sensing systems.
- We have started to learn bead production.
Week 9: First simulations, Skype with Paris Bettencourt, Chip fail
Wednesday, July 23rd
- We started to simulate diffusion experiments on a Comsol model. We consider cells will lay on agar for the moment.
We will have a chip with these four different channels 1 to 4 mm long. The model still has to be refined because at the moment it doesn't take into account internal/external concentrations of AHL.
- Our first mold didn't enable us to peal the PDMS correctly. We have to make it less deep.
- 5 of 8 regulator plasmids are done. We have to repeat construction of producer plasmids.
- Parameter estimation for integrases is not working at the moment.
- We skyped with Paris Bettencourt for human practice collaboration and are participating to their newsletter project.
Week 8 : Plasmdis assembly, First mold 3D printed
Wednesday, July 16th
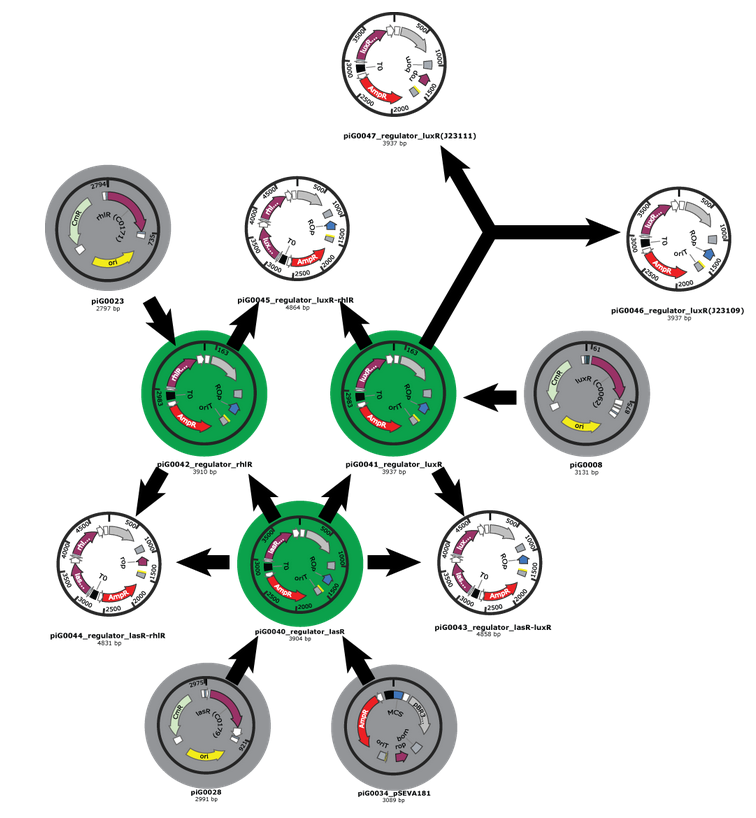
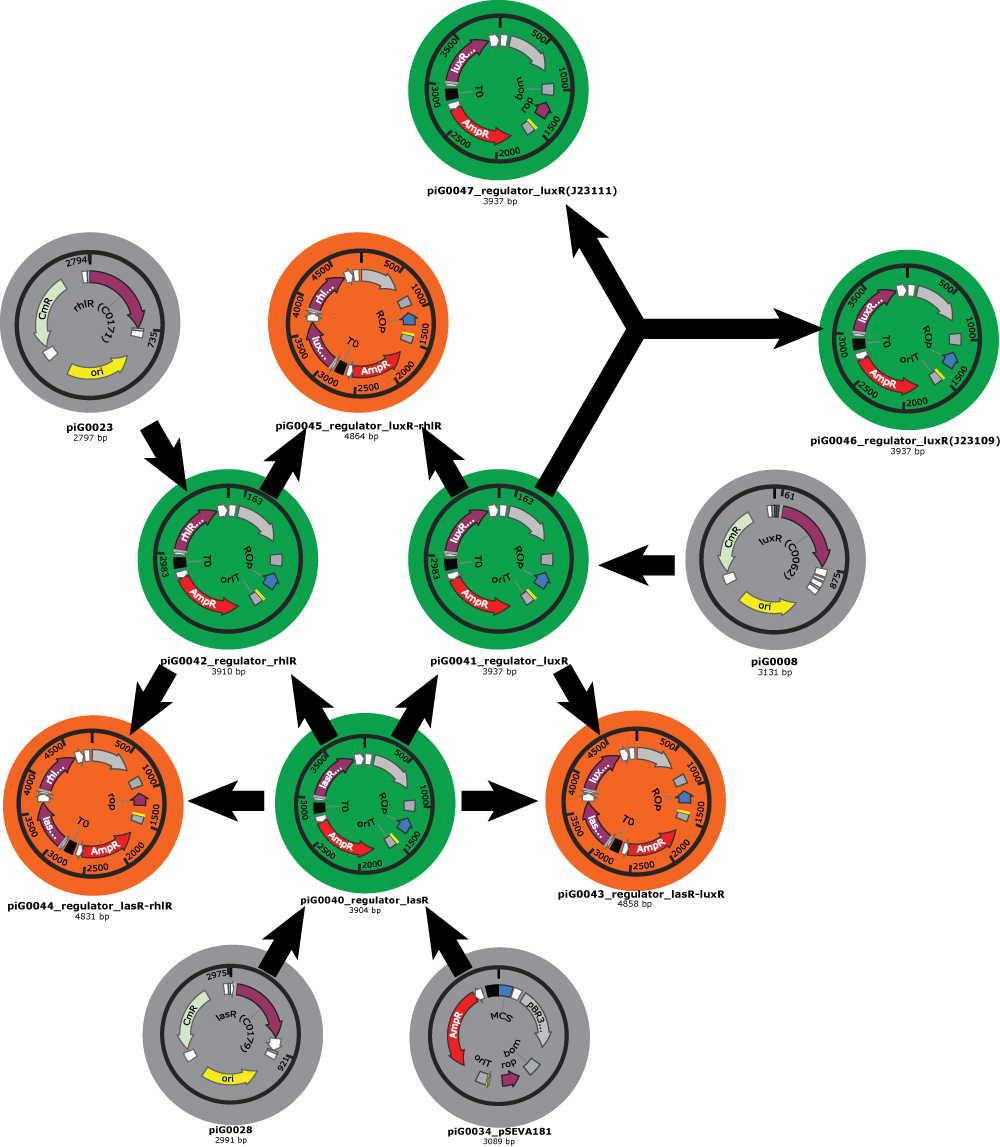
- Our first Gibson assemblies worked well. Regulator plasmids piG0040, piG0041 and piG0042 have been constructed :
- We printed our first mold for millifluidic chip :
- We simulated the whole model with and without riboswitch in Matlab with random parameters for integrases. We are still trying to estimate integrase parameters from the literature but it is very difficult.
Week 7: Human practice planning, Plasmid assembly running, Logo design
Wednesday, July 9th
- We have a logo !
- In the lab, we did some :
- Plasmid preparation
- Sequencing
- Digests
- Purification of backbone fragments needed for GA
- On the modeling side, we tried to estimate integrase parameters from the paper from Bonnet et al. [9], more particularly with the figure S4 :
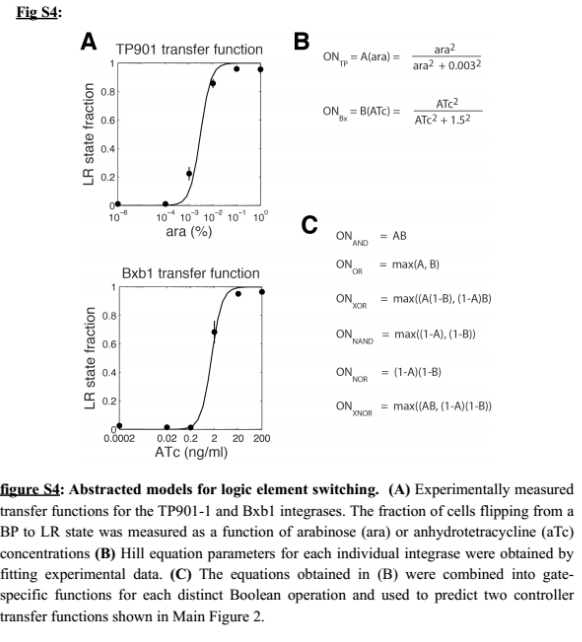
We are trying several strategies : minimization of the error function and Markov Chain Monte Carlo.
- We have a precise plan for our human practice project. We would like to study the emergence of complexity in many different fields and investigate how people deal with it. We will do this with interviews of experts in different fields, and with a survey for a wider outreach. We will also reach younger people by organizing talks in schools. We will finally give our own advice on the question by writing an essay on the subject and linking it to our experience with Mosaicoli.
Construction of the regulator plasmids
Thursday, July 5th
Construction of the lasR regulator plasmid (piG0040)
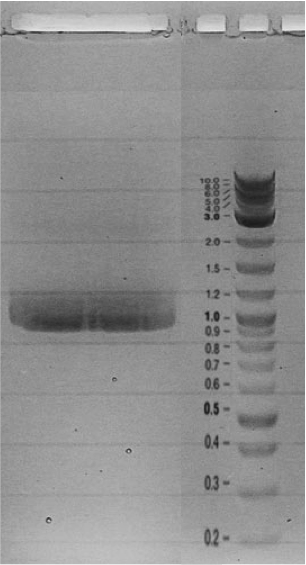
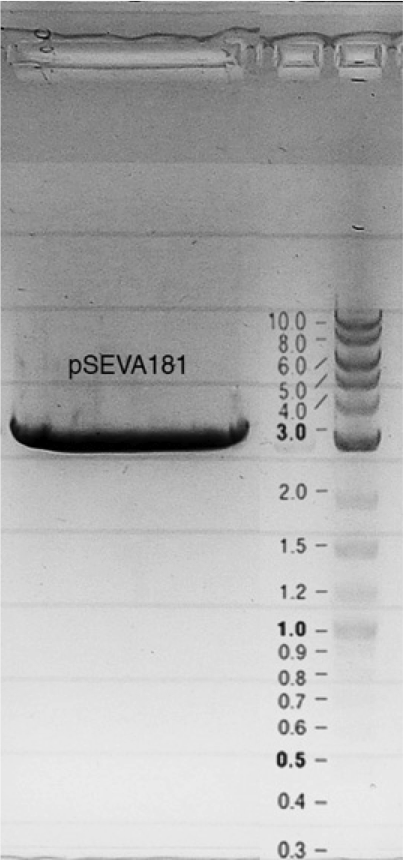
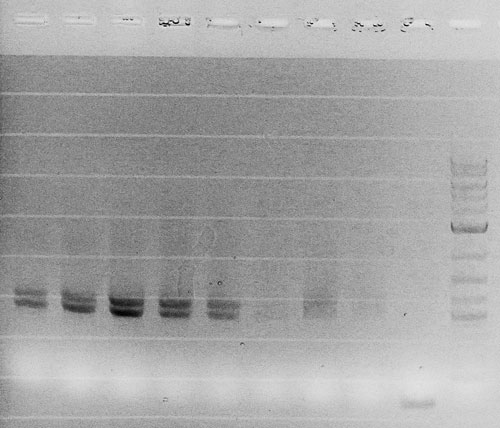
Competent cells were transformed with piG0028 (K553003, lasR) and selected on chloramphenicol-LB-plates. Plasmid DNA was extracted by performing a miniprep. The relevant plasmid sequence was sequenced by Microsynth using the primers oiG0001 and oiG0002. Amplification of lasR by PCR using oiG0003 and oiG0004 resulted in the fragment fiG0004 (1.0 kb). The vector piG0034 (pSEVA 181) was digested with the restriction enzymes HindIII and PacI to fiG0001 (3.0 kb). The two fragments, fiG0001 and fiG0004, were assembled using Gibbson assembly. Thus we used the plasmid backbone of piG0034 and lasR of piG0028 to construct the lasR regulator plasmid piG0040. Competent cells were transformed with piG0040 and selected on ampicillin-LB-plates. Colony PCR using the primers oiG0035 and oiG0036 was conducted to check the size of the inserted fragment (expected bands at 1.0 and 1.2 kb). The sequence was verified by Microsynth using the same primers.
Construction of the luxR regulator plasmid (piG0041)
Competent cells were transformed with piG0008 (F2620, luxR) and selected on chloramphenicol-LB-plates. Plasmid DNA was extracted by performing a miniprep. The relevant plasmid sequence was sequenced by Microsynth using the primers oiG0001 and oiG0002. Amplification of luxR by PCR using oiG0005 and oiG0006 resulted in the fragment fiG0005 (0.8 kb). The backbone of the vector piG0040 (lasR in pSEVA 181) was amplified by PCR using oiG0007 and oiG0008. This fragment, fiG0011 (3.2 kb), and fiG0005 were assembled using Gibbson assembly. Thus we formally replaced lasR by luxR to construct the luxR regulator plasmid piG0041. Competent cells were transformed with piG0041 and selected on ampicillin-LB-plates. Colony PCR using the primers oiG0035 and oiG0036 was conducted to check the size of the inserted fragment (expected bands at 1.1 and 1.2 kb). The sequence was verified by Microsynth using the same primers.
Construction of the rhlR regulator plasmid (piG0042)
Competent cells were transformed with piG0023 (C0171, rhlR) and selected on chloramphenicol-LB-plates. Plasmid DNA was extracted by performing a miniprep. The relevant plasmid sequence was sequenced by Microsynth using the primers oiG0001 and oiG0002. Amplification of rhlR by PCR using oiG0009 and oiG0010 resulted in the fragment fiG0006 (0.8 kb). The backbone of the vector piG0040 (lasR in pSEVA 181) was amplified by PCR using oiG0011 and oiG0012. This fragment, fiG0012 (3.2 kb), and fiG0006 were assembled using Gibbson assembly. Thus we formally replaced lasR by rhlR to construct the rhlR regulator plasmid piG0042. Competent cells were transformed with piG0042 and selected on ampicillin-LB-plates. Colony PCR using the primers oiG0035 and oiG0036 was conducted to check the size of the inserted fragment (expected bands at 1.1 and 1.2 kb). The sequence was verified by Microsynth using the same primers.
Construction of the luxR regulator plasmids with alternative constitutive promoters (piG0046 and piG0047)
The promoter site of the luxR regulator plasmid piG0041 was mutated by QuikChange site-specific mutagenesis to produce promoters of different strength. The primers oiG0031 and oiG0032 were used to establish piG0047, a plasmid with a promoter of intermediate strength (J23111). Analoguos the primers oiG0033 and oiG0034 were used to construct piG0046, a plasmid with a weak promoter (J23109). Competent cells were transformed with piG0046 and piG0047 and selected on ampicillin-LB-plates. The sequence was verified by Microsynth using the primers oig0035 and oiG0036.
Week 6: Project planning, Gibson assemblies planning, First Matlab simulations
Wednesday, July 2nd
- We have a clear organization of the different experiments we would like to perform.
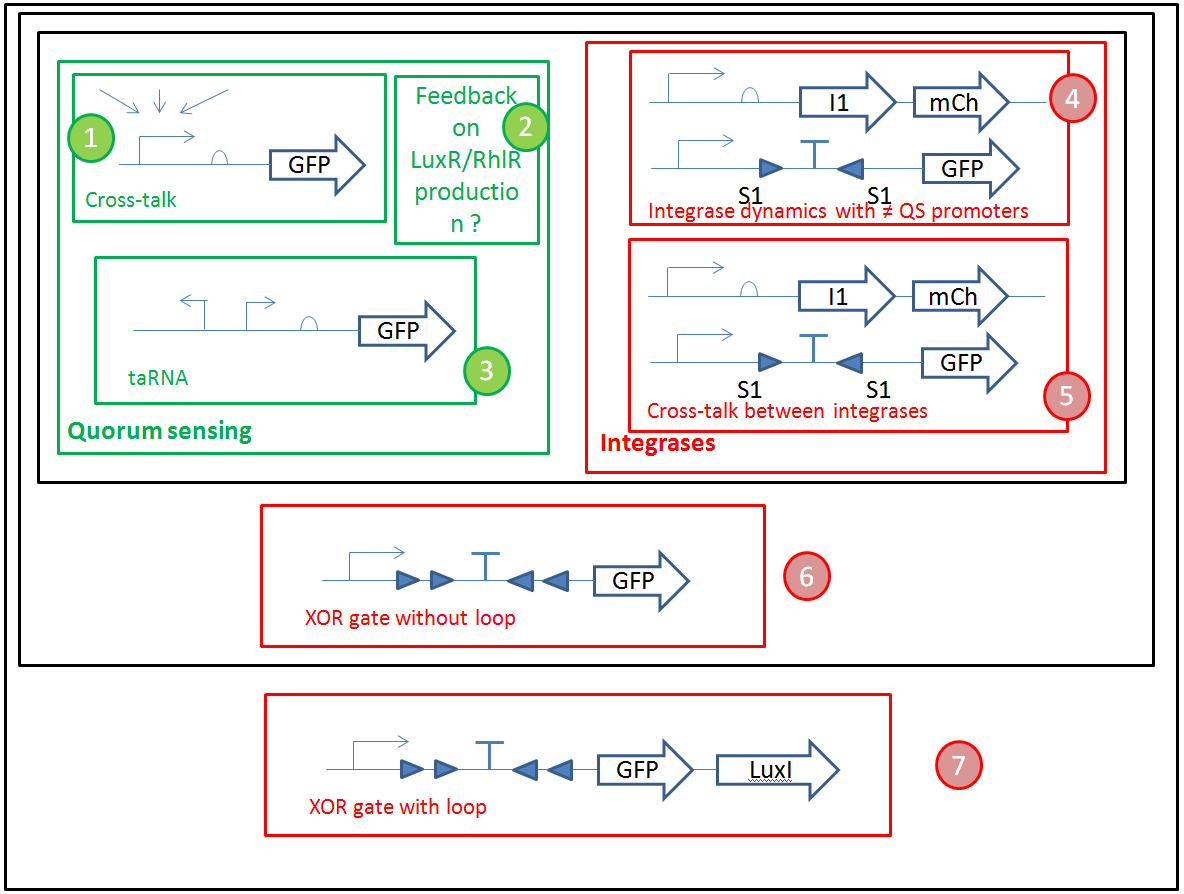
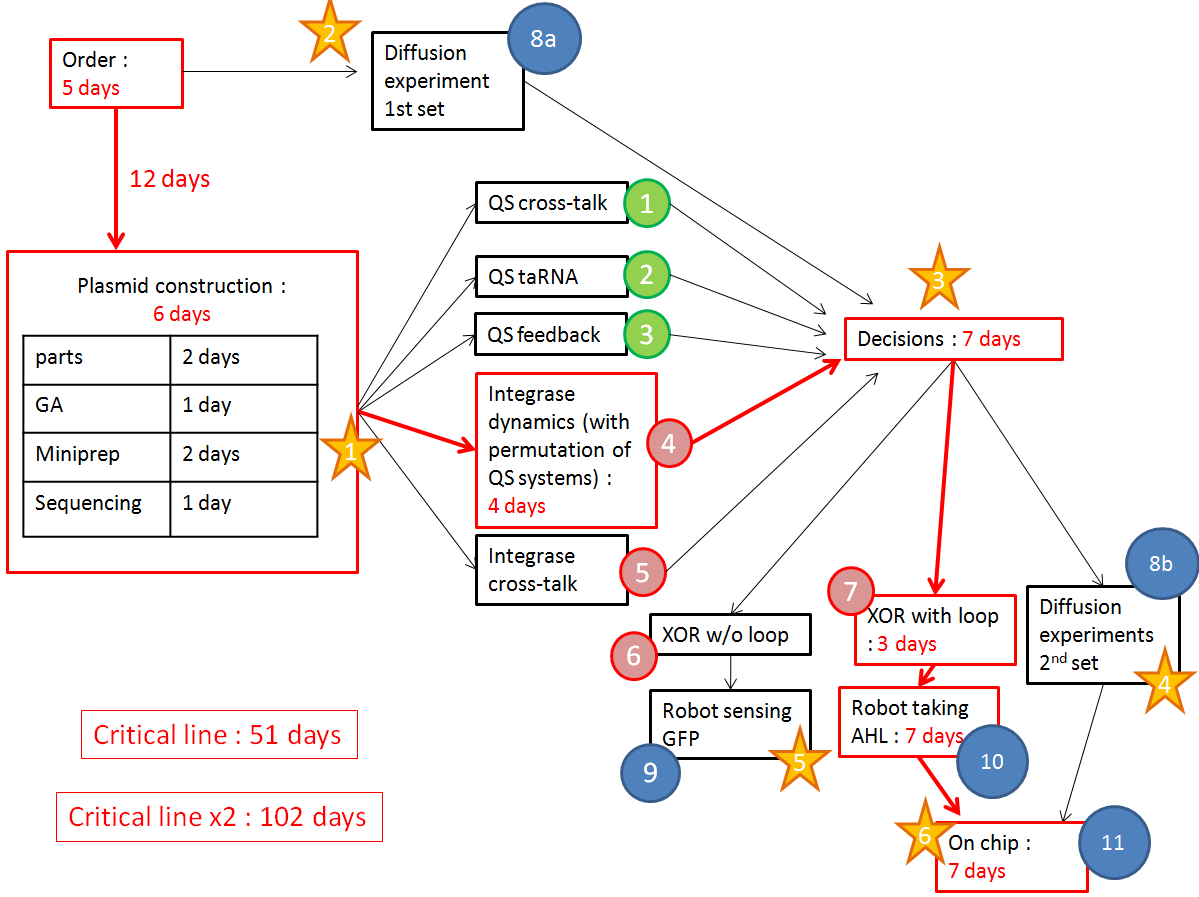
We want to optimize quorum sensing to have non-leaky non-cross-talking constructs. We want to prevent cross-talk between integrases and check their dynamics with different quorum sensing promoters. Then we will test the XOR gate without production of LuxI, to check how it works without the loop, and we will finally test our final construct with the loop, hoping that the delay between GFP production and the possible second switching due to AHL production is long enough to have enough fluorescence.
The red line is our critical timeline. Numbers of days are very optimistic,therefore we multiply the whole timeline by 2. The stars stand for modeling inputs.
- We have also clear plans for the Gibson assemblies to perform. As an example, here is the plan for regulator plasmids :
- We have a facebook page !
- We simulated quorum sensing without leakiness on Matlab, with parameters from the literature.
Week 5: Assembly strategy and steady states derivation
Wednesday, June 25th
- We will use Gibson assembly to assemble our constructs, and have now a construct assembly strategy. We know the list of experiments we want to carry (cross-talk between quorum sensing molecules, quorum sensing leakiness with or without riboswitch, cross-talk between integrases, integrase activity and logic gate behaviour) and the exact sequences of base units we will assemble (lux, rhl, las promoter and repressor units, reporter units, logic units with 3 different integrases, constitutive AHL production units for diffusion test, backbone units).
- From the modeling side, we calculated analytically the steady state of the integrase and terminator modules, and it appears that the deterministic model gives a binary steady state corresponding to an XOR gate. This is what we expected, it confirms that deterministic model in our case is interesting for the study of dynamics of the whole system, and not for steady state calculations. So we are now moving to dynamic studies.
Week 4: Improving plasmid design, Modularization of the whole model
Wednesday, June 18th
- We improved a little bit the plasmid design, determined which parts will be taken from the iGEM registry, and which parts we have to synthesize.
Sensor plasmids will be approx. 9.3kb long, and logic plasmids will be approx. 5,2kb long.
- From the modeling side, the whole model has been modularized and we have calculated and simulated steady state for the quorum sensing module.
- GeneScript, Microsynth, EraSynbio are willing to support us !
Modularization of the system
Sunday, June 15th
The idea of our system is to associate a quorum sensing part, which should be able to react to a certain concentration of input, with a integrase-base logic part, which should be able to derive a computation given the transformed inputs.
From a modeling point of view, it seemed obvious that we had to consider these two entities separately. That is why we chose to have a modularized approach, where we regrouped, on one hand, the equations corresponding to quorum sensing and, on the other hand, the ones corresponding to the integrase logic phenomena.
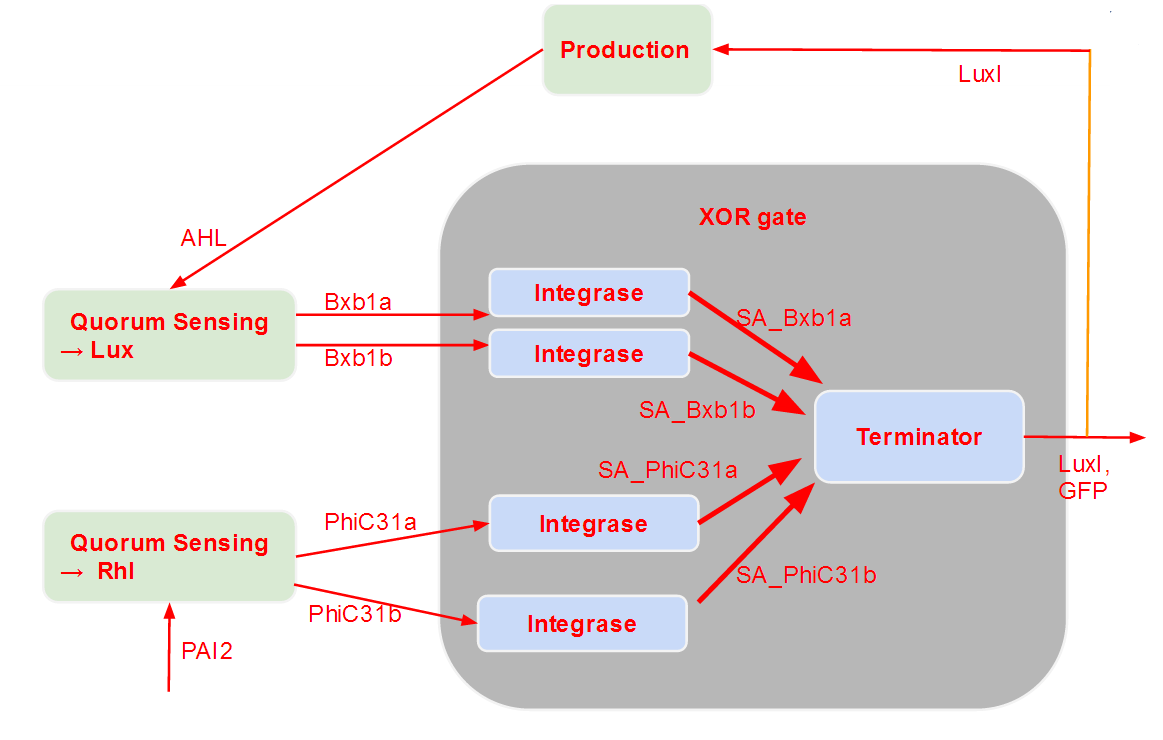
As the integrase module is not that well known, we further decomposed it into two successive parts. The first one only models the path integrases go through in order to bind to DNA. The second one models the switch behavior of the integrases (after they are bound to DNA) and describe the XOR logic of our system.
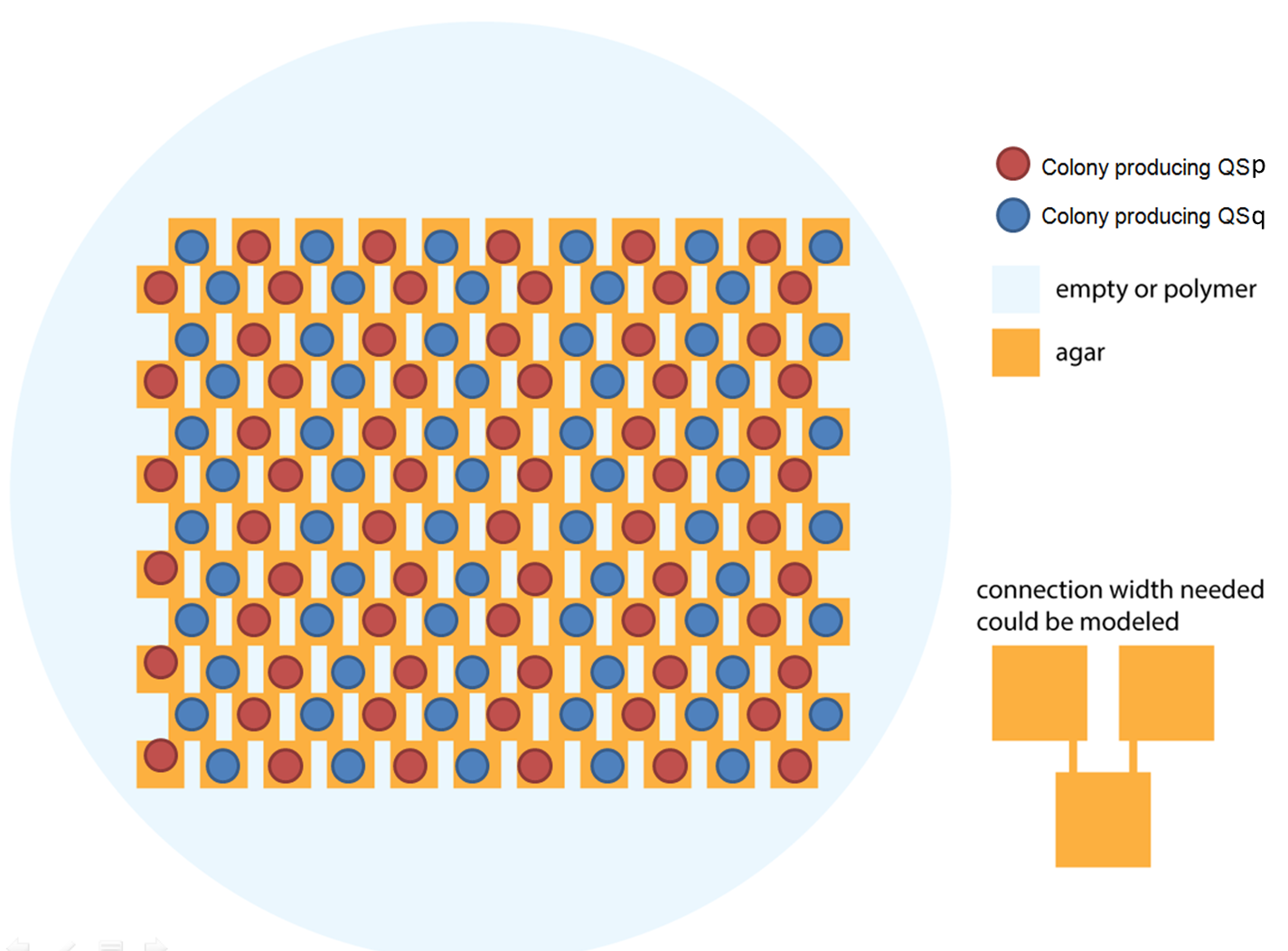
As we see, these modules are successive. After the logic module, we added a production module, which is responsible for the production of our outputs (GFP and an Homoserine Lactone that depends on the type of cell modeled). As one of our output is a messenger molecule, it has an influence on the quorum sensing module, which detects those molecules. Therefore, we have a feedback loop. This feedback loop comes from our choice to put only two different strains in our grid.
Week 3: Plasmid design started, Modeling started
Wednesday, June 11th
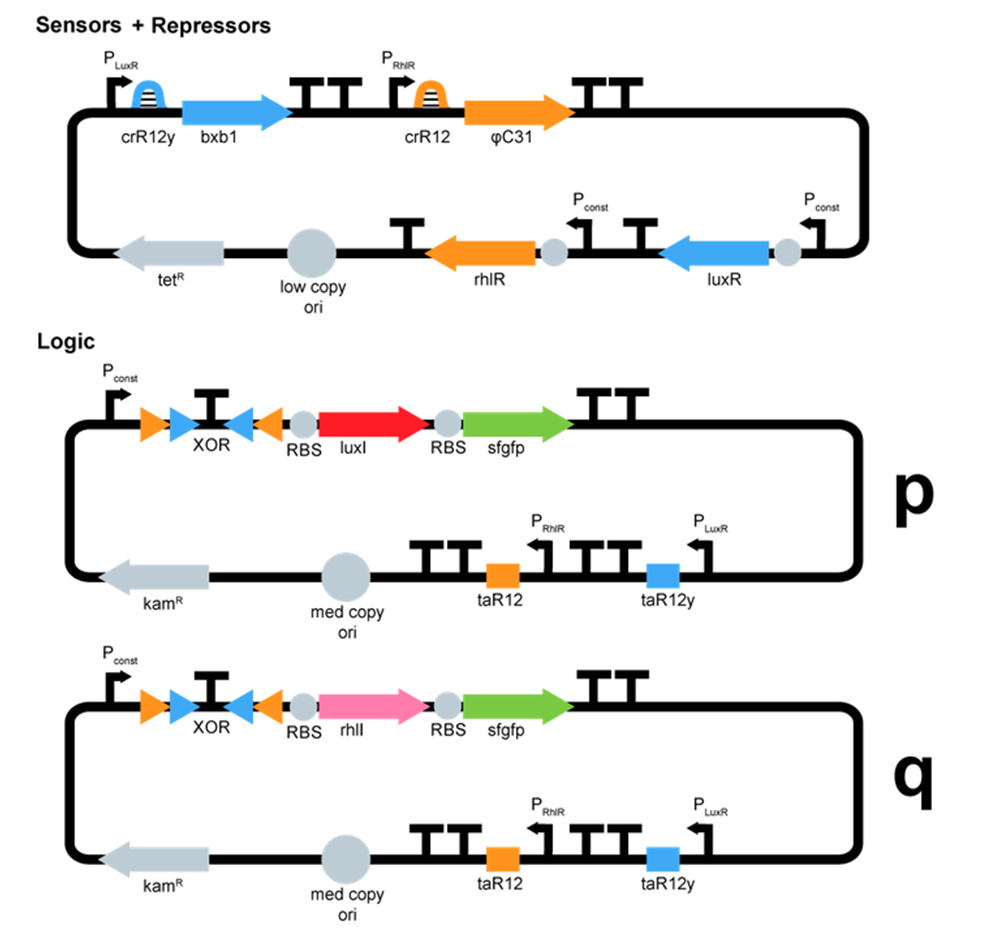
- We started plasmid design :
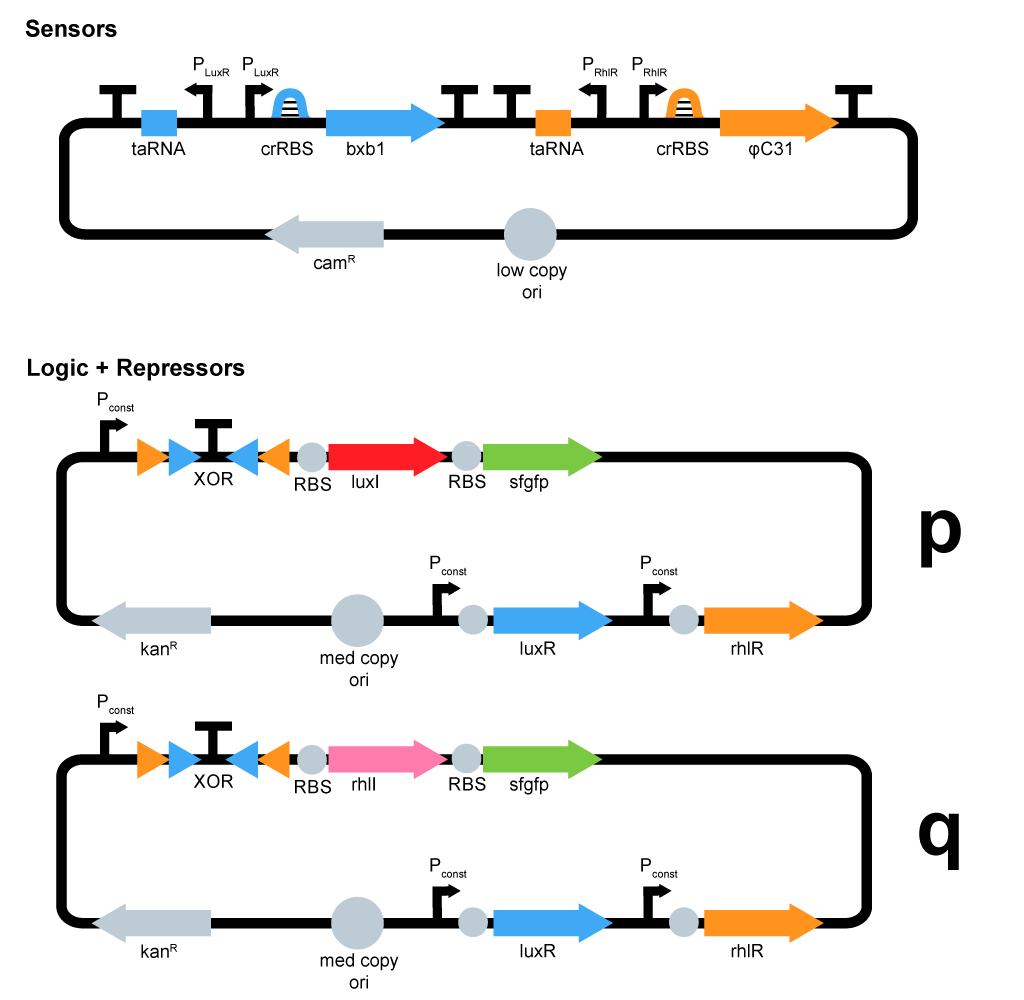
ΦC31 and Bxb1 are integrases. LuxR an RhlR are quorum sensing repressors. A riboswitch construct is placed around quorum sensing constructs to prevent leakiness of lux and rhl promoters. Type p colonies produce AHL by expressing the enzyme LuxI. Type q colonies produce Rhl by expressing the enzyme RhlI. In fact, we don't know yet which quorum sensing systems we will use. We will have to perform cross-talk experimetns in order to choose the ones that are the most orthogonal.
- We tried to print our first agar millifluidic chip : we printed it too small, and the printer had resolution problems.
- We wrote all reactions and found parameters from the literature for our model.
Week 2: Investigating microfluidics, Writing a modeling pipeline
Wednesday, June 4th
This week, we have talked with people from the microfluidics group and it came out that we should be able to use beads to encapsulate our cells and use a microfluidic chip. Therefore we will try to develop this possibility in parallel with the 3D-printed agar chip.
We have set up a modeling pipeline. We divided the modeling project into 3 parts :
- diffusion,
- parameter fitting,
- modeling of the genetic circuit itself, which comprises
- a deterministic model
- a stochastic model.
We know that the modeling challenges will be to model how integrases work, and to model the delay between reception of a quorum sensing signal and production of a quorum sensing signal by the receiver cell. Indeed, a colony could switch itself OFF when it should be ON, if it receives QS1 and produces QS2, for example. If a delay is present between reception of QS1 and production of QS2, the colony will produce GFP before it is switched OFF, and as GFP is stable enough, it will stay visible during a long time and the self-switching OFF won't be observed. This delay should be modeled.
Week 1 : Project selected
Wednesday, May 28th
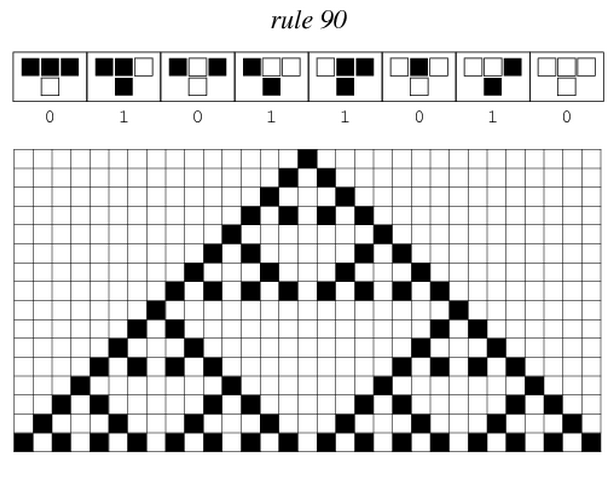
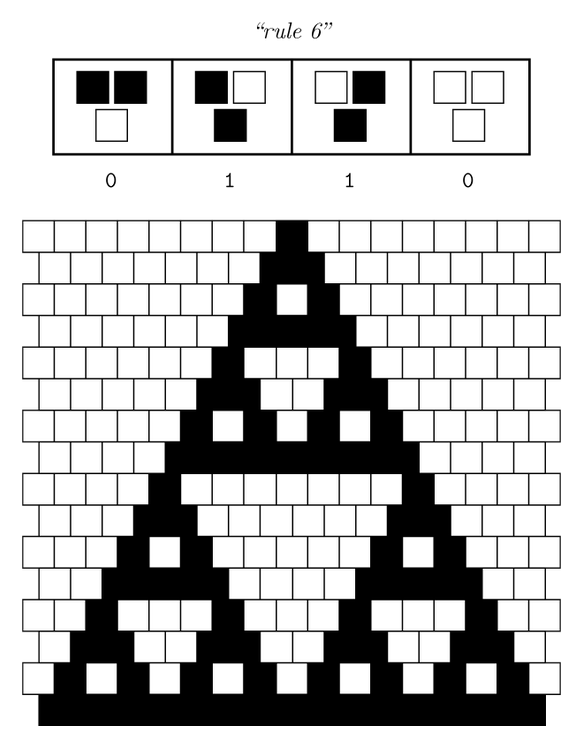
After more than one month of endless meetings and passionate debates, we finally chose the project that will keep us occupied in the next five months. From the beautiful pattern made by the Sierpinski triangles, we will focus on cellular automata and try to implement one.
Sierpinski triangles appear when the rule 90 is followed by every cell on the grid :
Ideally we will use a microfluidic chip. We could also use a 3D printed agar plate like this one to load the colonies. On this grid we can implement the rule 6, which is the simplification of rule 90 considered as a rule with 2 inputs : each cell computes a simple XOR gate of its two parents.
The logic part will be built with integrases and the colony-to-colony communication will use quorum sensing.
Every colony will receive two quorum sensing signals (QSp and QSq) from the two cells above it. These two signals trigger the production of two different integrases r and s in the colony. Integrases enable to build biological XOR logic gates by switching twice a terminator. Indeed, every integrase can switch the terminator only once. Thus if the colony produces only r or only s, the terminator is switched only once, so the terminator is OFF, and GFP and QS1 or QS2 are produced (depending on the colony). If the colony produces r and s, the terminator is switched twice, so it is ON and it blocks expression of GFP and of the quorum sensing molecule.
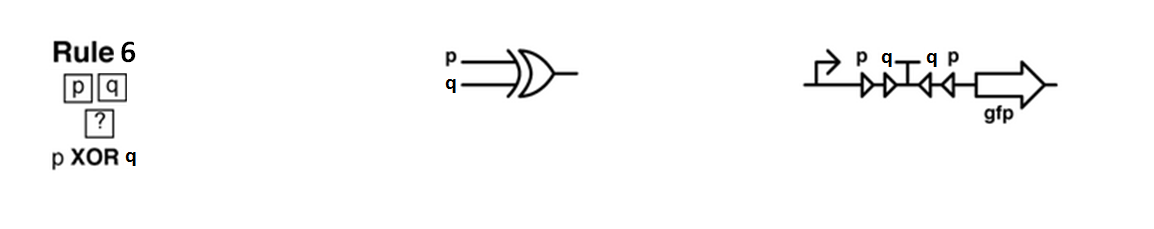
We need to :
- find orthogonal quorum sensing molecules and orthogonal integrases
- discuss with microfluidics experts to check if using microfluidics is possible and presents advantages in our case
- find possible parts in the registry for integrases, and design plasmids
BSSE Openhouse Day
Saturday, May 10th
Sharing our iGEM and synthetic biology interest with the public
On May 10th the public in Basel had the unique chance to get an insight into many different scientific laboratories and the work done there. It was the joint open house day of D-BSSE of ETH (Department of Biosystems Science and Engineering) and the Biozentrum of the University of Basel. The many different labs opened their doors to the public and many scientists were present to give interested people some details about their daily work. So did the ETH iGEM team 2014. The team was present with a poster showing the history of iGEM, the previous ETH iGEM teams with their projects and general information about synthetic biology. Additionally there was a slideshow giving a best-of photo collection of last years jamboree. The goal of this day was to inform the public about synthetic biology in general and specifically about the spirit and the many different projects of iGEM. Many people showed strong interest in truly student driven projects and are curious to follow our team wiki for the next months.
 "
"