Team:Bielefeld-CeBiTec
From 2014.igem.org
| (3 intermediate revisions not shown) | |||
| Line 124: | Line 124: | ||
<h6>Our project</h6> | <h6>Our project</h6> | ||
<p> | <p> | ||
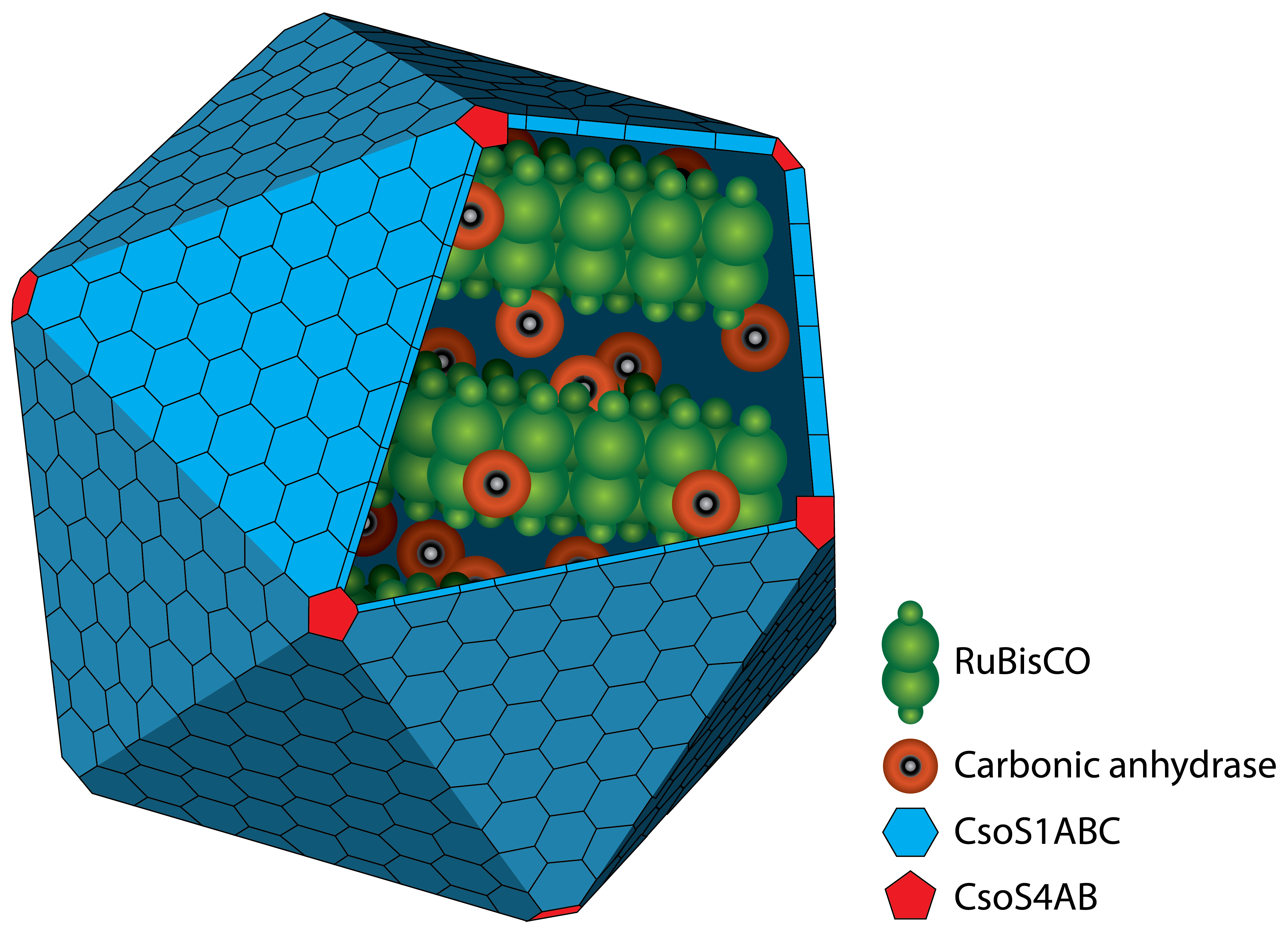
| - | + | Ecological power management is currently facing three challenges: the storage of electrical power, the development of renewable energy resources, which are not timely and locally synced with demand, and the increase of atmospheric carbon dioxide due to the use of fossil fuels. We address these challenges by implementing a proof of concept production of the biofuel isobutanol from carbon dioxide. In our project, a bacterial microcompartment from cyanobacteria, the carboxysome, was deployed in <i>Escherichia. coli</i> for carbon dioxide fixation under aerobic conditions. We further engineered <i>E. coli</i> to derive the energy for this process from electricity by implementing electron-mediator uptake and recycling. This ‘electricity’ pathway comprised proteins such as the furmarate reductase. Feasibility was analyzed in a self-constructed bioreactor. Ultimately, we established an isobutanol production pathway by heterologous expression of five genes from <i>E. coli</i>, <i>Bacillus subtilis</i> and <i>Lactococcus lactis</i>. | |
| + | </p><br> | ||
</div> | </div> | ||
</div> | </div> | ||
| Line 166: | Line 167: | ||
<tr><td><img src="https://static.igem.org/mediawiki/2014/2/2a/Bielefeld_CeBiTec_2014-10-17_Haken.png" width="50px"></td><td>Genome integration of <a href="https://2014.igem.org/Team:Bielefeld-CeBiTec/Results/rMFC" target="_blank">OprF</a></td></tr> | <tr><td><img src="https://static.igem.org/mediawiki/2014/2/2a/Bielefeld_CeBiTec_2014-10-17_Haken.png" width="50px"></td><td>Genome integration of <a href="https://2014.igem.org/Team:Bielefeld-CeBiTec/Results/rMFC" target="_blank">OprF</a></td></tr> | ||
<tr><td><img src="https://static.igem.org/mediawiki/2014/2/2a/Bielefeld_CeBiTec_2014-10-17_Haken.png" width="50px"></td><td>Functional <i>in-vitro</i> assay of <a href="https://2014.igem.org/Team:Bielefeld-CeBiTec/Results/CO2-fixation/RuBisCO" target"=_blank">RuBisCo</a></td></tr> | <tr><td><img src="https://static.igem.org/mediawiki/2014/2/2a/Bielefeld_CeBiTec_2014-10-17_Haken.png" width="50px"></td><td>Functional <i>in-vitro</i> assay of <a href="https://2014.igem.org/Team:Bielefeld-CeBiTec/Results/CO2-fixation/RuBisCO" target"=_blank">RuBisCo</a></td></tr> | ||
| - | <tr><td><img src="https://static.igem.org/mediawiki/2014/2/2a/Bielefeld_CeBiTec_2014-10-17_Haken.png" width="50px"></td><td>Development of an antibiotic-free selection system</td></tr> | + | <tr><td><img src="https://static.igem.org/mediawiki/2014/2/2a/Bielefeld_CeBiTec_2014-10-17_Haken.png" width="50px"></td><td>Development of an <a href="https://2014.igem.org/Team:Bielefeld-CeBiTec/Results/Biosafety" target="_blank">antibiotic-free selection system</a></td></tr> |
<tr><td><img src="https://static.igem.org/mediawiki/2014/2/2a/Bielefeld_CeBiTec_2014-10-17_Haken.png" width="50px"></td><td> | <tr><td><img src="https://static.igem.org/mediawiki/2014/2/2a/Bielefeld_CeBiTec_2014-10-17_Haken.png" width="50px"></td><td> | ||
Several <a href="https://2014.igem.org/Team:Bielefeld-CeBiTec/HumanPractice" target="_blank">human practice projects</a></td></tr> | Several <a href="https://2014.igem.org/Team:Bielefeld-CeBiTec/HumanPractice" target="_blank">human practice projects</a></td></tr> | ||
<tr><td><img src="https://static.igem.org/mediawiki/2014/2/2a/Bielefeld_CeBiTec_2014-10-17_Haken.png" width="50px"></td><td> | <tr><td><img src="https://static.igem.org/mediawiki/2014/2/2a/Bielefeld_CeBiTec_2014-10-17_Haken.png" width="50px"></td><td> | ||
| - | SYNENERGENE cooperation</td></tr> | + | <a href="https://2014.igem.org/Team:Bielefeld-CeBiTec/HumanPractice/Synenergene" target="_blank">SYNENERGENE cooperation</a></td></tr> |
<tr><td><img src="https://static.igem.org/mediawiki/2014/2/2a/Bielefeld_CeBiTec_2014-10-17_Haken.png" width="50px"></td><td> | <tr><td><img src="https://static.igem.org/mediawiki/2014/2/2a/Bielefeld_CeBiTec_2014-10-17_Haken.png" width="50px"></td><td> | ||
| - | Help another team</td></tr> | + | <a href="https://2014.igem.org/Team:Bielefeld-CeBiTec/HumanPractice/Wikisession" target="_blank">Help another team</a></td></tr> |
</table> | </table> | ||
</p> | </p> | ||
Latest revision as of 14:44, 28 November 2014
Welcome to our project
Our project
Ecological power management is currently facing three challenges: the storage of electrical power, the development of renewable energy resources, which are not timely and locally synced with demand, and the increase of atmospheric carbon dioxide due to the use of fossil fuels. We address these challenges by implementing a proof of concept production of the biofuel isobutanol from carbon dioxide. In our project, a bacterial microcompartment from cyanobacteria, the carboxysome, was deployed in Escherichia. coli for carbon dioxide fixation under aerobic conditions. We further engineered E. coli to derive the energy for this process from electricity by implementing electron-mediator uptake and recycling. This ‘electricity’ pathway comprised proteins such as the furmarate reductase. Feasibility was analyzed in a self-constructed bioreactor. Ultimately, we established an isobutanol production pathway by heterologous expression of five genes from E. coli, Bacillus subtilis and Lactococcus lactis.
Achievements
 | Design and construction of a rMFC |
 | Design and construction of a flow cell reactor |
 | Construction of an electrophilic E.coli strain |
 | Successful isobutanol production |
 | Construction of a functional microcompartment, the carboxysome |
 | Genome integration of OprF |
 | Functional in-vitro assay of RuBisCo |
 | Development of an antibiotic-free selection system |
 | Several human practice projects |
 | SYNENERGENE cooperation |
 | Help another team |
 "
"