Team:Aachen/Project/2D Biosensor
From 2014.igem.org
(→Achievements) |
(→Achievements) |
||
| (153 intermediate revisions not shown) | |||
| Line 18: | Line 18: | ||
<a href="https://2014.igem.org/Team:Aachen/Project/2D_Biosensor#biosensorpoo" style="color:black"> | <a href="https://2014.igem.org/Team:Aachen/Project/2D_Biosensor#biosensorpoo" style="color:black"> | ||
<div class="team-item team-info" style="width:214px;height:214px;" > | <div class="team-item team-info" style="width:214px;height:214px;" > | ||
| - | <div class="menukachel">Principle of Operation</div> | + | <div class="menukachel" style="top:32%;line-height:1.5em;">Principle of Operation</div> |
<!-- <br/><br/> | <!-- <br/><br/> | ||
<b>Principle of Operation</br> | <b>Principle of Operation</br> | ||
| Line 31: | Line 31: | ||
<a href="https://2014.igem.org/Team:Aachen/Project/2D_Biosensor#biosensordevelopment" style="color:black"> | <a href="https://2014.igem.org/Team:Aachen/Project/2D_Biosensor#biosensordevelopment" style="color:black"> | ||
<div class="team-item team-info" style="width:214px;height:214px;" > | <div class="team-item team-info" style="width:214px;height:214px;" > | ||
| - | <div class="menukachel">Development & Optimization</div> | + | <div class="menukachel" style="top:32%;line-height:1.5em;">Development & Optimization</div> |
<!-- <br/><br/> | <!-- <br/><br/> | ||
<b>Principle of Operation</br> | <b>Principle of Operation</br> | ||
| Line 44: | Line 44: | ||
<a href="https://2014.igem.org/Team:Aachen/Project/2D_Biosensor#biosensorachievements" style="color:black"> | <a href="https://2014.igem.org/Team:Aachen/Project/2D_Biosensor#biosensorachievements" style="color:black"> | ||
<div class="team-item team-info" style="width:214px;height:214px;" > | <div class="team-item team-info" style="width:214px;height:214px;" > | ||
| - | <div class="menukachel">Achievements</div> | + | <div class="menukachel" style="top:40%">Achievements</div> |
<!-- <br/><br/> | <!-- <br/><br/> | ||
<b>Principle of Operation</br> | <b>Principle of Operation</br> | ||
| Line 57: | Line 57: | ||
<a href="https://2014.igem.org/Team:Aachen/Project/2D_Biosensor#biosensoroutlook" style="color:black"> | <a href="https://2014.igem.org/Team:Aachen/Project/2D_Biosensor#biosensoroutlook" style="color:black"> | ||
<div class="team-item team-info" style="width:214px;height:214px;" > | <div class="team-item team-info" style="width:214px;height:214px;" > | ||
| - | <div class="menukachel">Outlook</div> | + | <div class="menukachel" style="top:40%;">Outlook</div> |
<!-- <br/><br/> | <!-- <br/><br/> | ||
<b>Principle of Operation</br> | <b>Principle of Operation</br> | ||
| Line 78: | Line 78: | ||
<span class="anchor" id="biosensorpoo"></span> | <span class="anchor" id="biosensorpoo"></span> | ||
| - | ''Cellock Holmes'' is designed upon a SynBio approach comprising a '''two-dimensional biosensor and a measurement unit'''. The two-dimensional biosensor is devised to recognize quorum sensing molecules secreted by the pathogen cells and to generate a distinct fluorescence signal; while the measurement device recognizes and analyzes the produced signal. | + | ''Cellock Holmes'' is designed upon a SynBio approach comprising a '''two-dimensional biosensor and a measurement unit'''. The two-dimensional biosensor is devised to recognize quorum sensing molecules secreted by the pathogen cells and to generate a distinct fluorescence signal; while the measurement device recognizes and analyzes the produced signal. On the molecular side, we use the '''[https://2014.igem.org/Team:Aachen/Project/FRET_Reporter REACh construct]''' to transform regular ''E. coli'' cells into ''Cellocks''. |
| - | + | ||
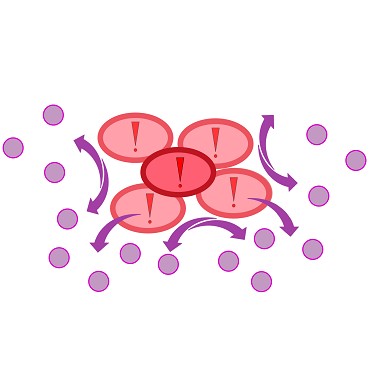
{{Team:Aachen/Figure|Aachen 17-10-14 The basics of quorum sensing ipo.png||title=The principle of quorum sensing|subtitle=Microorganisms can sense the presence of their own kind based on quorum sensing which is a form of chemical communication. Depending on their cell density, quorum sensing allows these cells to activate or deactivate certain gene expression cascades (Waters and Bassler, 2005) for a specific function.|width=900px}} | {{Team:Aachen/Figure|Aachen 17-10-14 The basics of quorum sensing ipo.png||title=The principle of quorum sensing|subtitle=Microorganisms can sense the presence of their own kind based on quorum sensing which is a form of chemical communication. Depending on their cell density, quorum sensing allows these cells to activate or deactivate certain gene expression cascades (Waters and Bassler, 2005) for a specific function.|width=900px}} | ||
Our '''sensor cells, ''Cellocks'', are immobilized in agar chips'''. To make the chips, we mix the ''Cellocks'' with liquid LB agar. | Our '''sensor cells, ''Cellocks'', are immobilized in agar chips'''. To make the chips, we mix the ''Cellocks'' with liquid LB agar. | ||
| - | In the course of our project, we designed a casting mold specifically for the production of our agar chips. When the agar has cooled down, the chips are cut out of the mold and are ready to use. Storage of the readily usable sensor chips is possible for up to | + | In the course of our project, we designed a casting mold specifically for the production of our agar chips. When the agar has cooled down, the chips are cut out of the mold and are ready to use. Storage of the readily usable sensor chips is possible for up to two days at 4°C when using LB medium or up to five days if TB medium is used. A detailed description of the sensor chip manufacturing can be found in our [https://2014.igem.org/Team:Aachen/Notebook/Protocols/detection Protocols] section. |
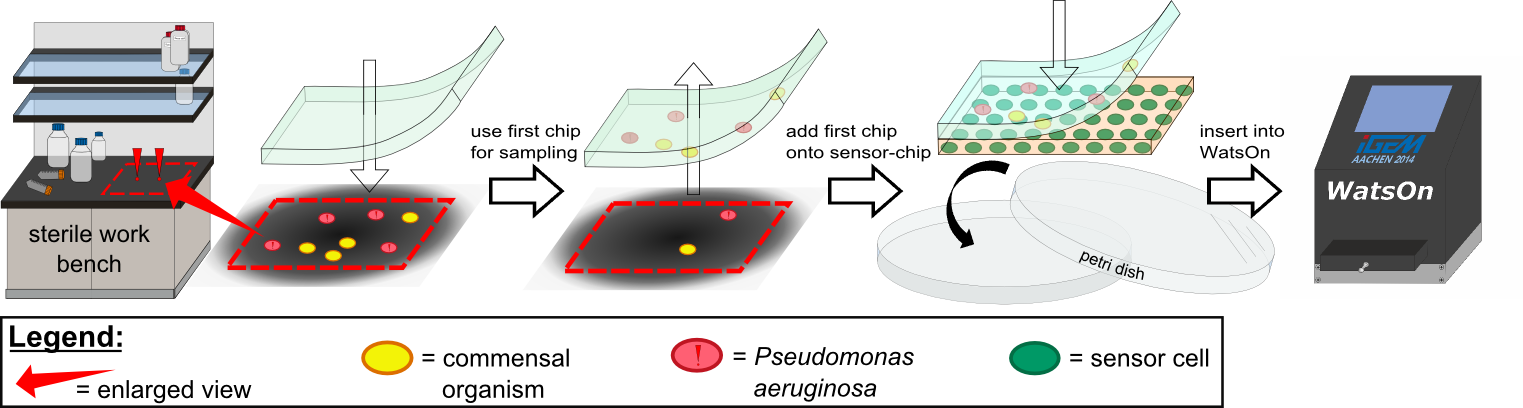
{{Team:Aachen/Figure|Aachen 14-10-14 Flowsheet OD-device part1 ipo.png|title=Assay to detect ''P. aeruginosa'' using ''Cellock Holmes''|subtitle=This flow sheet shows the procedure to sample and detect ''P. aeruginosa'': A sampling chip is briefly put onto the potentially contaminated surface, added onto one of our sensor chips and inserted into ''WatsOn''.|width=900px}} | {{Team:Aachen/Figure|Aachen 14-10-14 Flowsheet OD-device part1 ipo.png|title=Assay to detect ''P. aeruginosa'' using ''Cellock Holmes''|subtitle=This flow sheet shows the procedure to sample and detect ''P. aeruginosa'': A sampling chip is briefly put onto the potentially contaminated surface, added onto one of our sensor chips and inserted into ''WatsOn''.|width=900px}} | ||
| - | Using ''Cellock Holmes'', we developed a simple assay to detect ''P. aeruginosa''. | + | Using ''Cellock Holmes'', we developed a simple assay to detect ''P. aeruginosa''. Initially, a so called sampling chip is placed on a solid surface that is potentially contaminated with the pathogen. Subsequently, the sampling chip is removed from the surface and put onto one of our sensor chips. Theorectically, the sensor chips could be directly used for sampling, however, this was avoided in our project to '''match [https://2014.igem.org/Team:Aachen/Safety biosafety regulations]''' and to prevent the spread of genetically modified organisms (GMOs) into the environment. The two layered chip-stack is then put into a petri dish which is inserted into our measurement device [https://2014.igem.org/Team:Aachen/Project/Measurement_Device ''WatsOn''] for evalutation. |
| - | + | ||
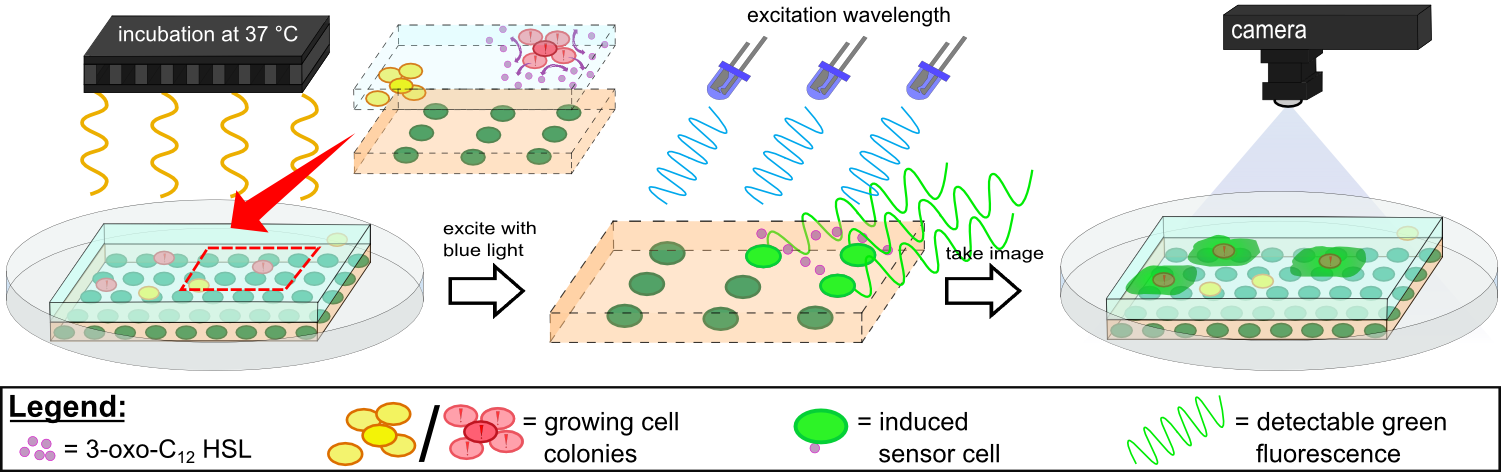
{{Team:Aachen/Figure|Aachen 14-10-14 Flowsheet OD-device part2 ipo.png|title=Mode of action inside ''WatsOn''|subtitle=Chips are incubated at 37°C to stimulate cell growth and then illuminated with blue light to excite fluorescence. A picture is taken and analyzed for fluorescence signals using the software ''Measurarty''.|width=900px}} | {{Team:Aachen/Figure|Aachen 14-10-14 Flowsheet OD-device part2 ipo.png|title=Mode of action inside ''WatsOn''|subtitle=Chips are incubated at 37°C to stimulate cell growth and then illuminated with blue light to excite fluorescence. A picture is taken and analyzed for fluorescence signals using the software ''Measurarty''.|width=900px}} | ||
| - | Inside ''WatsOn'', the chips are incubated at 37°C and the sampled populations of microorganisms attached on the sampling chip start to grow and multiply. During incubation the chips can be '''illuminated with blue light''' at any time, and a '''photo of the chips''' is taken. The '''software ''Measurarty''''' then analyzes any fluorescent signal. | + | Inside ''WatsOn'', the chips are incubated at 37°C and the sampled populations of microorganisms attached on the sampling chip start to grow and multiply. During incubation the chips can be '''illuminated with blue light''' at any time, and a '''photo of the chips''' is taken. The '''software ''Measurarty''''' then analyzes any fluorescent signal. ''P. aeruginosa'' secrets an increasing number of quorum sensing molecules that are recognized by ''Cellocks'', thereby producing a fluorescence signal. For detection of ''P. aeruginosa'', we focused on a quorum sensing molecule called N-3-oxo-dodecanoyl-L-homoserine lactone (for short: 3-oxo-C<sub>12</sub>-HSL), which is involved in virulence regulation of ''P. aeruginosa'' (Jimenez, Koch, Thompson et al., 2012). The incorporation of the 3-oxo-C<sub>12</sub>-HSL detection system into the ''Cellocks'' is explained in detail in the [https://2014.igem.org/Team:Aachen/Project/FRET_Reporter REACh Construct] section. |
| Line 118: | Line 116: | ||
<span class="anchor" id="biosensordevelopment"></span> | <span class="anchor" id="biosensordevelopment"></span> | ||
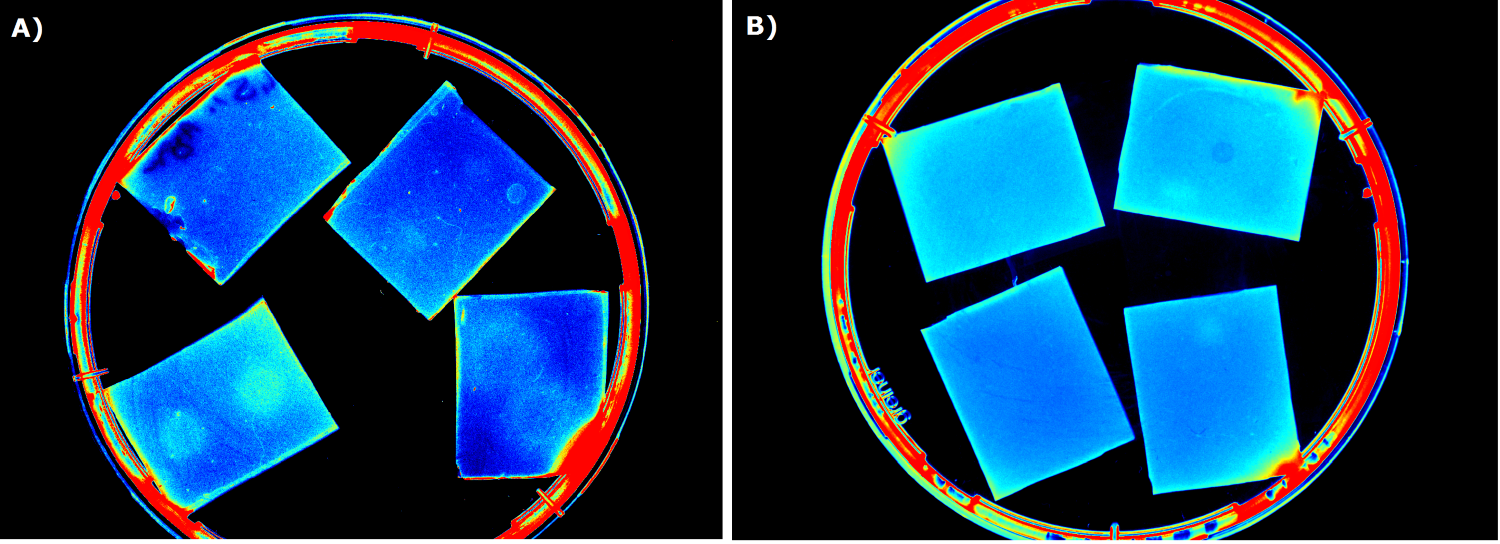
| - | {{Team:Aachen/FigureFloat|Aachen_ILOV_GFP_HM_1,5h.png|title=iLOV and GFP in the Gel Doc<sup>TM</sup>|subtitle=Sensor cells producing iLOV (A) and GFP (B) 1.5 h after induction.|left|width=500px}} | + | {{Team:Aachen/FigureFloat|Aachen_ILOV_GFP_HM_1,5h.png|title=iLOV and GFP in the Gel Doc<sup>TM</sup>|subtitle=Sensor cells producing iLOV (K1319042, A) and GFP (B) 1.5 h after induction.|left|width=500px}} |
=== Equipment and medium selection === | === Equipment and medium selection === | ||
| - | Our first approach (before developing our own device) was to use the Molecular Imager® Gel Doc™ XR+ from BIO-RAD in our lab to detect fluorescence | + | Our first approach (before developing our own device) was to use the Molecular Imager® Gel Doc™ XR+ from BIO-RAD in our lab to detect fluorescence. This device uses UV and white light illuminators. However, only two different filters were available for the excitation light wavelength, which resulted in very limited possibilities for the excitation of fluorescent molecules. For example, it was possible to detect the expression of iLOV (K1319042) in our sensor chips, but not the expression of GFP. Hence, the '''Gel Doc™ was not suitable for our project'''. |
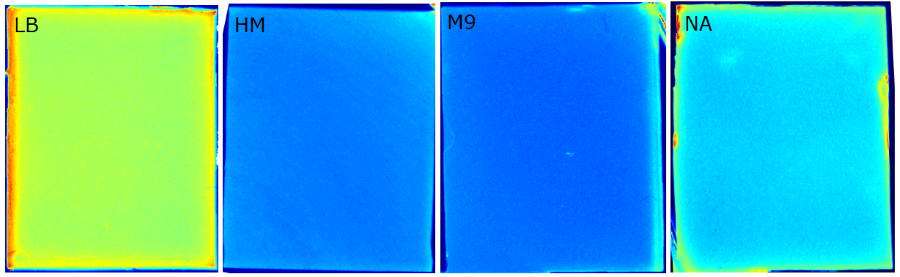
{{Team:Aachen/FigureFloat|Aachen_Chip_medium_geldoc.png|title=Differend medium in the Gel Doc™|subtitle=complex media exhibited high background fluorescence while less back- ground fluorescence was observed with the minimal media (HM, M9, NA).|right|width=500px}} | {{Team:Aachen/FigureFloat|Aachen_Chip_medium_geldoc.png|title=Differend medium in the Gel Doc™|subtitle=complex media exhibited high background fluorescence while less back- ground fluorescence was observed with the minimal media (HM, M9, NA).|right|width=500px}} | ||
| - | {{Team:Aachen/FigureFloat|Aachen_5days_K131026_neb_tb_1,5h.jpg |title=Testing our chips' shelf-life|subtitle= Chips of [http://parts.igem.org/Part:BBa_K131026 K131026] in NEB were stored | + | {{Team:Aachen/FigureFloat|Aachen_5days_K131026_neb_tb_1,5h.jpg |title=Testing our chips' shelf-life|subtitle= Chips of [http://parts.igem.org/Part:BBa_K131026 K131026] in NEB were stored five days at 4°C. The right chip was induced with 0.2 µL of 500 µg/mL HSL and an image was taken after 1.5 h.|left|width=500px}} |
| - | We tested different media ( | + | We tested different media (LB, TB, M9, NA and HM) for the preparation of our sensor chips. The medium compositions can be found in the [https://2014.igem.org/Team:Aachen/Notebook/Protocols Protocols] section. We screened for an optimized medium composition to minimize background fluorescence and to enhance cell growth. The results of the analysis are presented in the table below. Due to the low background fluorescence in ''WatsOn'' and the excellent cell growth, we '''chose LB medium''' over the other tested media for sensor chip manufacturing. |
<center> | <center> | ||
| Line 137: | Line 135: | ||
</center> | </center> | ||
| - | + | Another set of experiments were conducted to test the '''long-time storage''' of the sensor chips. We varied the glycerol content of the chips as well as the storage temperature. Storage at -20°C resulted in the loss of our sensor cells. Adding 5-10% (v/v) glycerol ensured survival of the sensor cells, but resulted in the loss of fluorescence ability. Hence, we concluded that long-time storage of the sensor chips at -20°C is not possible under the tested conditions. However, the 'ready-to-use' sensor chips can be kept at at 4°C for two days when using LB medium, and storage at this temperature for 5 days is possible with chips made from TB medium. | |
<!--Regarding the medium used for our sensor chips, LB medium showed a high background fluorescence when exposed to UV light in the Gel Doc. Surprisingly, the background fluorescence resulting from the LB medium was too high to detect a signal emitted by our sensor cells. Hence, minimal media (NA, M9, Hartman (HM)) was used to minimize background fluorescence, but this approach resulted in less to no growth of our sensor cells. In our device ''WatsOn'', optimized wavelengths of 450 nm and 480 nm were used for excitation of iLOV and GFP, respectively. When exposed to either excitation wavelength TB medium, which is basically an improved LB medium and highly supports cell growth, showed strong background fluorescence in our own device. High background fluorescence was also observed for TB medum when using the Gel Doc. In contrast to the Gel Doc LB medium showed minimal fluorescence in our device ''WatsOn'' and no difficulties in cultivation of our ''Cellocks'' were observed. Because of the reduced fluorescence compared to TB medium when using ''Watson'' for sensor chip evaluation and because of sufficient cultivation conditions for our 'Cellocks'' LB medium was chosen over TB mediium for sensor chip manufacturing. --> | <!--Regarding the medium used for our sensor chips, LB medium showed a high background fluorescence when exposed to UV light in the Gel Doc. Surprisingly, the background fluorescence resulting from the LB medium was too high to detect a signal emitted by our sensor cells. Hence, minimal media (NA, M9, Hartman (HM)) was used to minimize background fluorescence, but this approach resulted in less to no growth of our sensor cells. In our device ''WatsOn'', optimized wavelengths of 450 nm and 480 nm were used for excitation of iLOV and GFP, respectively. When exposed to either excitation wavelength TB medium, which is basically an improved LB medium and highly supports cell growth, showed strong background fluorescence in our own device. High background fluorescence was also observed for TB medum when using the Gel Doc. In contrast to the Gel Doc LB medium showed minimal fluorescence in our device ''WatsOn'' and no difficulties in cultivation of our ''Cellocks'' were observed. Because of the reduced fluorescence compared to TB medium when using ''Watson'' for sensor chip evaluation and because of sufficient cultivation conditions for our 'Cellocks'' LB medium was chosen over TB mediium for sensor chip manufacturing. --> | ||
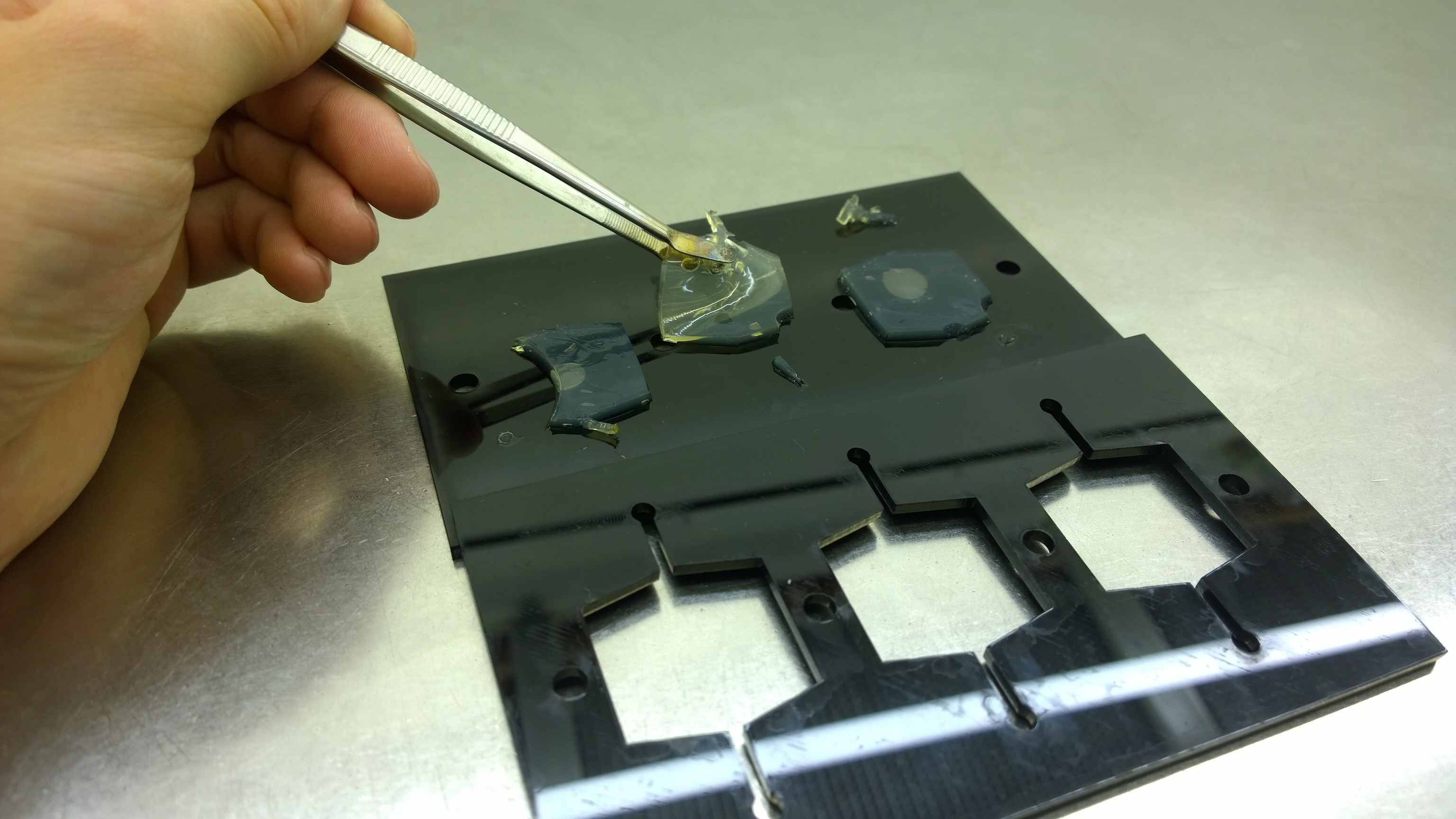
{{Team:Aachen/FigureFloat|Aachen_2_chipform.jpg|title=Sensor chip manufacturing using the closed mold|subtitle=When injecting the liquid agar into a closed mold we encounter problems due to frequent bubble formation.|left|width=500px}} | {{Team:Aachen/FigureFloat|Aachen_2_chipform.jpg|title=Sensor chip manufacturing using the closed mold|subtitle=When injecting the liquid agar into a closed mold we encounter problems due to frequent bubble formation.|left|width=500px}} | ||
| - | === Optimal | + | |
| - | For the sensor chip manufacturing, agarose was preferred over agar | + | === Optimal Agarose Concentration for Sensor Chip Manufacturing === |
| + | For the sensor chip manufacturing, agarose was preferred over agar because of the uniform linkage between molecules that results in a better chip homogeneity. In addition, agarose reduced diffusion of the inducer molecules through the chip. A reduction in diffusion is essential for the formation of distinct fluorescent spots on the sensor chips. | ||
| + | |||
{{Team:Aachen/FigureFloat|Aachen_Final_chipform.jpg|title=The finalized chip mold|subtitle=An open casting mold was found to be optimal for sensor chip manufacturing, because this approach was fast, easy to handle and generated a reproducible chip quality.|left|width=500px}} | {{Team:Aachen/FigureFloat|Aachen_Final_chipform.jpg|title=The finalized chip mold|subtitle=An open casting mold was found to be optimal for sensor chip manufacturing, because this approach was fast, easy to handle and generated a reproducible chip quality.|left|width=500px}} | ||
| - | === Optimal | + | === Optimal Chip Configuration === |
| - | Several approaches were tested for the production of agarose-based sensor chips with reproducible quality. The first approach was to cast every sensor chip individually. To achieve a plain chip surface | + | Several approaches were tested for the production of agarose-based sensor chips with reproducible quality. The first approach was to cast every sensor chip individually. To achieve a plain chip surface, a requirement for high quality images, we casted the sensor chips between two microscope slides. However, this approach was not adequate because the agar was too liquid and leaked from the microscope slides. In a second approach, we designed a closed mold into which liquid agar is injected using a pipette, but we encountered a high number of bubbles in the resulting chips. Bubbles in the sensor chips interfered with fluorescence evaluation. Finally, we tried an open casting mold. Once solidified, we cut the agar along precast indentations in the casting mold to form the chips. An advantage of the open mold is the ability to simultaneously produce nine sensor chips while the surface tension of the liquid agar ensures a plane chip surface. |
| - | === Induction of the | + | === Induction of the Sensor Chips === |
| - | + | To test our molecular constructs, we simulated the presence of ''P. aeruginosa'' by using IPTG or 3-oxo-C<sub>12</sub>-HSL. Initial experiments showed that diffusion of the inducers hinder the formation of distinct fluorescent spots. Through this set of experiments we determined that the best compromise between diffusion and spot intensity is an induction volume of 2.0 µL for IPTG and 0.2 µL for HSL. Furthermore, detection of growing ''P. aeruginosa'' based on secreted HSLs was possible using the [http://parts.igem.org/Part:BBa_K131026 K131026] construct. The experiments for optimizing the induction of our sensor chips are described in more detail in the [https://2014.igem.org/Team:Aachen/Project/2D_Biosensor#biosensorachievements Achievements] section. | |
| + | === Negative Control === | ||
| + | To ensure that the fluorescence signal resulted from the sensor construct and not from the medium or ''E. coli'' cells themselves, [http://parts.igem.org/Part:BBa_B0015 B0015] in NEB10β cells was used as negative control during sensor chip induction with IPTG, HSL and ''P. aeruginosa''. | ||
| + | |||
| + | {{Team:Aachen/Figure|Aachen_B0015_IPTG_HSL_Pseudomonas.png|title=B0015 in NEB10β was used as a negativ control|subtitle=Induction with A) 0.2 µL of 100 mM IPTG, image taken after 2.5 h; B) 0.2 µL of 500 µg/mL 3-oxo-C<sub>12</sub>-HSL, image after 2.5 h; C) with five spots of ''Pseudomonas aeruginosa'' liquid culture on the left and one big spot on the right, image taken after 2 h.|width=900px}} | ||
| + | |||
{{Team:Aachen/BlockSeparator}} | {{Team:Aachen/BlockSeparator}} | ||
| Line 156: | Line 161: | ||
<span class="anchor" id="biosensorachievements"></span> | <span class="anchor" id="biosensorachievements"></span> | ||
| - | We developed and optimized a 2D biosensor, which | + | We developed and optimized a 2D biosensor, which is able to detect IPTG, 3-oxo-C{{sub|12}}-HSL and living ''Pseudomonas aeruginosa'' cells. |
| - | + | ||
| - | + | ||
| + | {{Team:Aachen/FigureFloat|Aachen_K1319042_Platereader.gif|title=Testing K1319042 in our sensor chips|subtitle=Sensor chips based on [http://parts.igem.org/Part:BBa_K1319042 K1319042] were investigated for fluorescence using a plate reader. Blue color indicates the absence of fluorescence, while red color indicates fluorescence. The upper chip was not induced, while the lower chip was induced with IPTG (2.0 µL, 100mM).|width=260px}} | ||
=== Testing our Sensor Chips in a Plate Reader === | === Testing our Sensor Chips in a Plate Reader === | ||
| - | |||
| - | To establish a prove of principle we used our construct [http://parts.igem.org/Part:BBa_K1319042 K1319042] an IPTG inducible iLOV. | + | To establish a prove-of-principle for our sensor chip design, we used our construct [http://parts.igem.org/Part:BBa_K1319042 K1319042], an IPTG inducible iLOV. ''E. coli'' cells carrying the this construct were introduced into sensor chips and fluorescence was measured every 15 minutes after induction with 2 µL of 100 mM IPTG. The results are displayed on the left. |
| + | We observed a distinct difference in fluorescence between the non-induced chip (top) and the induced chip (bottom). The middle of the bottom chip started to exhibit a clear and visible fluorescence that increased over time and spread outwards. The top chip, however, also showed an increase in the measured fluorescence over time which was primarily due to the leaky promoter and background fluorescence. | ||
| - | + | {{Team:Aachen/FigureFloatRight|Aachen_K131026_Platereader.gif|title=Testing K131026 in our sensor chips|subtitle=Sensor chips based on [http://parts.igem.org/Part:BBa_K131026 K131026] were investigated for fluorescence using a plate reader. Blue color indicates the absence of fluorescence, while red color indicates fluorescence. The lower chip was induced with with 3-oxo-C{{sub|12}}-HSL (0.2 µL, 500 µg/mL).|width=360px}} | |
| - | + | === Detecting 3-oxo-C{{sub|12}}-HSL with Sensor Chips === | |
| + | In an initial attempt to detect 3-oxo-C{{sub|12}}-HSL, we incorporated the [http://parts.igem.org/Part:BBa_K131026 K131026] construct generated by the 2008 iGEM Team Calgary in our sensor chips. This construct generates a fluorescent signal based on GFP in presence of 3-oxo-C{{sub|12}}-HSL molecules produced by ''P. aeruginosa'' during quorum sensing (Jimenez, Koch, Thompson et al., 2012). First, we tested the construct by direct induction with 3-oxo-C{{sub|12}}-HSL (0.2 µL, 500 µg/mL). The fluorescence measurement was taken every 15 minutes with an excitation wavelength of 496 nm and an emission wavelength of 516 nm. The results of this test are shown on the right. | ||
| + | A distinct fluorescence signal was observed on the induced chip (bottom) compared to the non-induced chip (top). | ||
| + | Fluorescence started in the middle of the chip, the point of induction, and then extended outwards while growing in intesnity. The basal level of fluorescence was attributed to leakiness of the promoter and general background fluorescence of growing ''E. coli'' cells. In the induced chip (bottom), the background fluorescence was lower than in the non-induced chip (top) because the signal masked the noise. The difference between the induced and non-induced chips indicates a clear response to the HSL and proofed the ability of our 2D sensor chip design to detect HSLs produced by ''Pseudomonas aeruginosa''. | ||
| + | {{Team:Aachen/FigureFloat|Aachen_I746909_slower_reduced.gif|title=IPTG-inducible superfolder GFP (I746909) in sensor chips|subtitle=Expression of superfolder GFP ([http://parts.igem.org/Part:BBa_I746909 I746909]) was induced by the addition of IPTG (2 µL, 100mM) on the right chip. The left chip was not induced.|width=480px}} | ||
| + | === Detecting IPTG with Sensor Chips === | ||
| + | The clip displayed on the left side shows the construct [http://parts.igem.org/Part:BBa_I746909 I746909] from the 2007 iGEM Team Cambridge which expresses super folder GFP under the control of a T7 promoter in combination with our 2D sensor chip technology. The [http://parts.igem.org/Part:BBa_I746909 I746909] construct was introduced into BL21(DE3) cells to make the expression IPTG-inducible since the genome of BL21(De3) contains the T7 RNA Polymerase under the control of a lacI promoter. | ||
| + | While the left chip does not show visible fluorescence, the right chip exhibits a strong fluorescence signal. This proves the ability of our sensor chip technology to detect IPTG. The fluorescence response is also high enough to be detected and analyzed by our measurement device ''WatsOn''. | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | + | {{Team:Aachen/FigureFloatRight|Aachen_K131026_HSLdetection_slow.gif|title=Detection of 3-oxo-C{{sub|12}}-HSL with K131026|subtitle=0.2 µL of 3-oxo-C{{sub|12}}-HSL was placed in the middle of a sensor chip based on [http://parts.igem.org/Part:BBa_K131026 K131026] followed by incubation at 37°C in ''WatsOn''.|width=480px}} | |
| - | {{Team:Aachen/ | + | |
| - | + | ===Detecting the 3-oxo-C{{sub|12}}-HSL with K131026 in our Sensor Chips using ''WatsOn''=== | |
| - | The | + | The next step towards the final goal of detecting living cells of ''Pseudomonas aeruginosa'' was to reproduce the detection of 3-oxo-C{{sub|12}}-HSL, already established in the plate reader, in our own [https://2014.igem.org/Team:Aachen/Project/Measurement_Device ''WatsOn''] device. Therefore, we again used ''E. coli'' BL21(DE3) cells carrying [http://parts.igem.org/Part:BBa_K131026 K131026] and induced with 0.2 µL 3-oxo-C{{sub|12}}-HSL of a concentration of 500 µg/mL. In the clip displayed on the left, the right chip was induced and - as a negative control - the left chip was not induced. Pictures were taken every four minutes. |
| + | The result was a clear replication of the success of the plate reader experiment. The induced chip showed a clear fluorescence response eminating from the center, where the induction with HSL took place. This demonstrated the ability of not only our sensor chip technology but also our measurement device ''WatsOn to successfully'' detect 3-oxo-C{{sub|12}}-HSL. | ||
| Line 197: | Line 204: | ||
| + | {{Team:Aachen/FigureFloat|Aachen_K131026_Pseudomonas_aeruginosa_detection.gif|title=Detection of ''Pseudomonas aeruginosa'' with K131026|subtitle=Direct detection of ''Pseudomonas aeruginosa'' on sensor chips based on [http://parts.igem.org/Part:BBa_K131026 K131026].|width=480px}} | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
===Detecting ''Pseudomonas aeruginosa'' with K131026 in our Sensor Chip with ''WatsOn''=== | ===Detecting ''Pseudomonas aeruginosa'' with K131026 in our Sensor Chip with ''WatsOn''=== | ||
| - | |||
| - | |||
| - | |||
| - | + | After establishing the successful detection of 3-oxo-C{{sub|12}}-HSLs with our sensor chips the next step was to detect living cells of ''Pseudomonas aeruginosa'' with our measurement device ''WatsOn''. Therefore sensor chips based on [http://parts.igem.org/Part:BBa_K131026 K131026] were prepared and the right chip was induced with 0.2 µL of a ''Pseudomonas aeruginosa'' culture while the left chip was not induced (Detection of 3-oxo-C12 HSL with K131026, displayed below). On the induced chip, a clear fluorescence signal was visible in response to ''P. aeruginosa''. The fluorescence signal emerged outward from the induction point and showed a significant difference to the non-induced chip. The results clearly demonstrate the ability of our sensor chip technology and our measurement device ''WatsOn'' to detect ''P. aeruginosa''! | |
{{Team:Aachen/BlockSeparator}} | {{Team:Aachen/BlockSeparator}} | ||
| Line 225: | Line 218: | ||
<span class="anchor" id="biosensoroutlook"></span> | <span class="anchor" id="biosensoroutlook"></span> | ||
| - | + | We are '''committed to improve''' our sensor chip platform. The current technique of using a simple agarose chip is not sufficient to collect all microorganisms from the sampled surface. Therefore, the aim is to improve the sampling chip by using a different, more adhesive material. | |
| - | Furthermore, diffusion in the sensor chips will be reduced to '''limit the spread of the fluorescence signal'''. Currently, the fluorescence spot grows | + | Furthermore, diffusion in the sensor chips will be reduced to '''limit the spread of the fluorescence signal'''. Currently, the fluorescence spot grows beyond the point of induction and makes it difficult to differentitate between multiple points of induction. By introducing diffusion barriers into our chips, the growth of the fluorescence spots might be reduced, thus enabling the detection of multiple sources of fluorescence lying close together. |
| - | Additionally, the application of our sensor chips in combination with our ''WatsOn'' device is currently being evaluated for the detection of signals other than fluorescence. '''Detecting bio- and chemiluminescence''' has been identified as an area of potential future application. | + | Additionally, the application of our sensor chips in combination with our ''WatsOn'' device is currently being evaluated for the detection of signals other than fluorescence. '''Detecting bio- and chemiluminescence''' has been identified and will be investigated as an area of potential future application. |
| Line 238: | Line 231: | ||
* Waters, C. M., & Bassler, B. L. (2005). QUORUM SENSING: Cell-to-Cell Communication In Bacteria. Annual Review of Cell and Developmental Biology, 21(1), 319-346. Available online at http://www.annualreviews.org/doi/full/10.1146/annurev.cellbio.21.012704.131001. | * Waters, C. M., & Bassler, B. L. (2005). QUORUM SENSING: Cell-to-Cell Communication In Bacteria. Annual Review of Cell and Developmental Biology, 21(1), 319-346. Available online at http://www.annualreviews.org/doi/full/10.1146/annurev.cellbio.21.012704.131001. | ||
| - | * Jimenez, P. N., Koch, G., Thompson, J. A., Xavier, K. B., Cool, R. H., & Quax, W. J. (2012). The Multiple Signaling Systems Regulating Virulence in Pseudomonas aeruginosa. Microbiology and Molecular Biology Reviews, 76(1), 46-65. Available online at http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3294424/#B63. | + | * Jimenez, P. N., Koch, G., Thompson, J. A., Xavier, K. B., Cool, R. H., & Quax, W. J. (2012). The Multiple Signaling Systems Regulating Virulence in ''Pseudomonas aeruginosa''. Microbiology and Molecular Biology Reviews, 76(1), 46-65. Available online at http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3294424/#B63. |
| + | |||
{{Team:Aachen/Footer}} | {{Team:Aachen/Footer}} | ||
Latest revision as of 03:43, 18 October 2014
|
|
|
|
|
|
 "
"