Team:Aachen/Collaborations/Kaiser-Karls-Gymnasium
From 2014.igem.org
(→Lessons 5 & 6 - Glowing Vanilla Pudding) |
(→Lessons 5 & 6 - Glowing Vanilla Pudding) |
||
| Line 163: | Line 163: | ||
<html><a style="text-align: center; display: block;" href="http://igem.rwth-aachen.de/wordpress/wp-content/uploads/2014/06/10371107_641111112633015_62538188_o.jpg"><img src="http://igem.rwth-aachen.de/wordpress/wp-content/uploads/2014/06/10371107_641111112633015_62538188_o-1024x682.jpg" alt="Quorum Sensing Folie" width="590" height="392" class="aligncenter size-large wp-image-186" /></a></html> | <html><a style="text-align: center; display: block;" href="http://igem.rwth-aachen.de/wordpress/wp-content/uploads/2014/06/10371107_641111112633015_62538188_o.jpg"><img src="http://igem.rwth-aachen.de/wordpress/wp-content/uploads/2014/06/10371107_641111112633015_62538188_o-1024x682.jpg" alt="Quorum Sensing Folie" width="590" height="392" class="aligncenter size-large wp-image-186" /></a></html> | ||
| - | {{Team:Aachen/Figure|Aachen School | + | {{Team:Aachen/Figure|Aachen School Vfischeri.png|||width=590px}} |
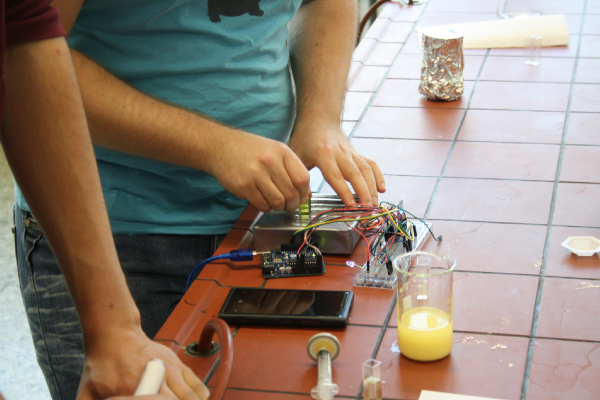
Meanwhile the powder had dissolved in the water. Excess powder accumulated at the bottom of the beaker. Using a syringe, the students suck 2mL of supernatant out of the beaker, and press it through a filter into a cuvette. A part of our project involves the development of a fluorescence measurement device named ''Cellock Holmes''. Each group of students at a time places its cuvette in the ''Cellock Holmes'' prototype, and notes down the values displayed on the cellphone display. Of course, each group also measures the fluorescence of a positive (pure riboflavin in water) and a negative (chalk dust in water) control. | Meanwhile the powder had dissolved in the water. Excess powder accumulated at the bottom of the beaker. Using a syringe, the students suck 2mL of supernatant out of the beaker, and press it through a filter into a cuvette. A part of our project involves the development of a fluorescence measurement device named ''Cellock Holmes''. Each group of students at a time places its cuvette in the ''Cellock Holmes'' prototype, and notes down the values displayed on the cellphone display. Of course, each group also measures the fluorescence of a positive (pure riboflavin in water) and a negative (chalk dust in water) control. | ||
| Line 169: | Line 169: | ||
<html><a style="text-align: center; display: block;" href="http://igem.rwth-aachen.de/wordpress/wp-content/uploads/2014/06/10172978_641110589299734_1681172065_o.jpg"><img src="http://igem.rwth-aachen.de/wordpress/wp-content/uploads/2014/06/10172978_641110589299734_1681172065_o-1024x682.jpg" alt="Measuring with ''Cellock Holmes''" width="590" height="392" class="aligncenter size-large wp-image-187" /></a></html> | <html><a style="text-align: center; display: block;" href="http://igem.rwth-aachen.de/wordpress/wp-content/uploads/2014/06/10172978_641110589299734_1681172065_o.jpg"><img src="http://igem.rwth-aachen.de/wordpress/wp-content/uploads/2014/06/10172978_641110589299734_1681172065_o-1024x682.jpg" alt="Measuring with ''Cellock Holmes''" width="590" height="392" class="aligncenter size-large wp-image-187" /></a></html> | ||
| - | {{Team:Aachen/Figure|Aachen School | + | {{Team:Aachen/Figure|Aachen School experiment.png|||width=590px}} |
While the other groups wait for their turn, their supervisors explain the background of this experiment: The electrons in some molecules change into energy states when irradiated with electromagnetic waves. When returning to the normal state, the electrons dissipate excess energy, also in the form of electromagnetic radiation. This process is called fluorescence. Vanilla pudding contains the molecule riboflavin (vitamin B2). When riboflavin is irradiated with blue light, the molecule fluoresces green. The intensity of the green light is visually recorded by our device. Special software processes the data and the measured value is displayed on the cellphone connected to the device via Bluetooth. We will discuss the results of the experiment next class. | While the other groups wait for their turn, their supervisors explain the background of this experiment: The electrons in some molecules change into energy states when irradiated with electromagnetic waves. When returning to the normal state, the electrons dissipate excess energy, also in the form of electromagnetic radiation. This process is called fluorescence. Vanilla pudding contains the molecule riboflavin (vitamin B2). When riboflavin is irradiated with blue light, the molecule fluoresces green. The intensity of the green light is visually recorded by our device. Special software processes the data and the measured value is displayed on the cellphone connected to the device via Bluetooth. We will discuss the results of the experiment next class. | ||
Revision as of 21:20, 17 October 2014
|
|
|
|
|
|
|
 "
"