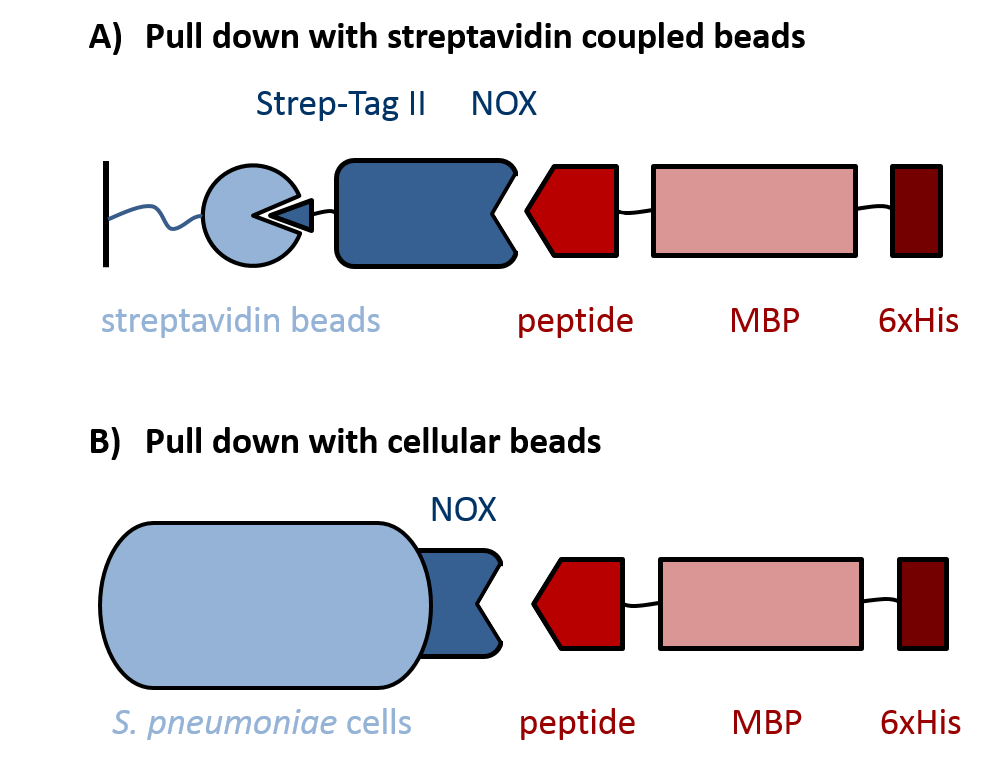
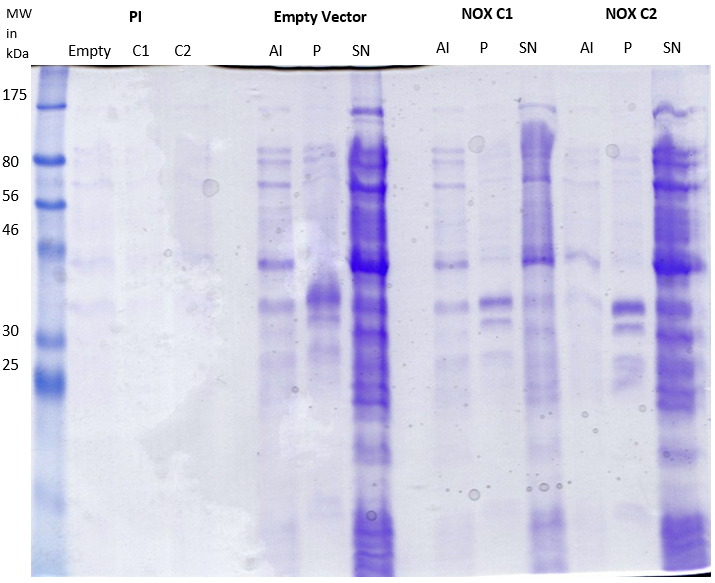
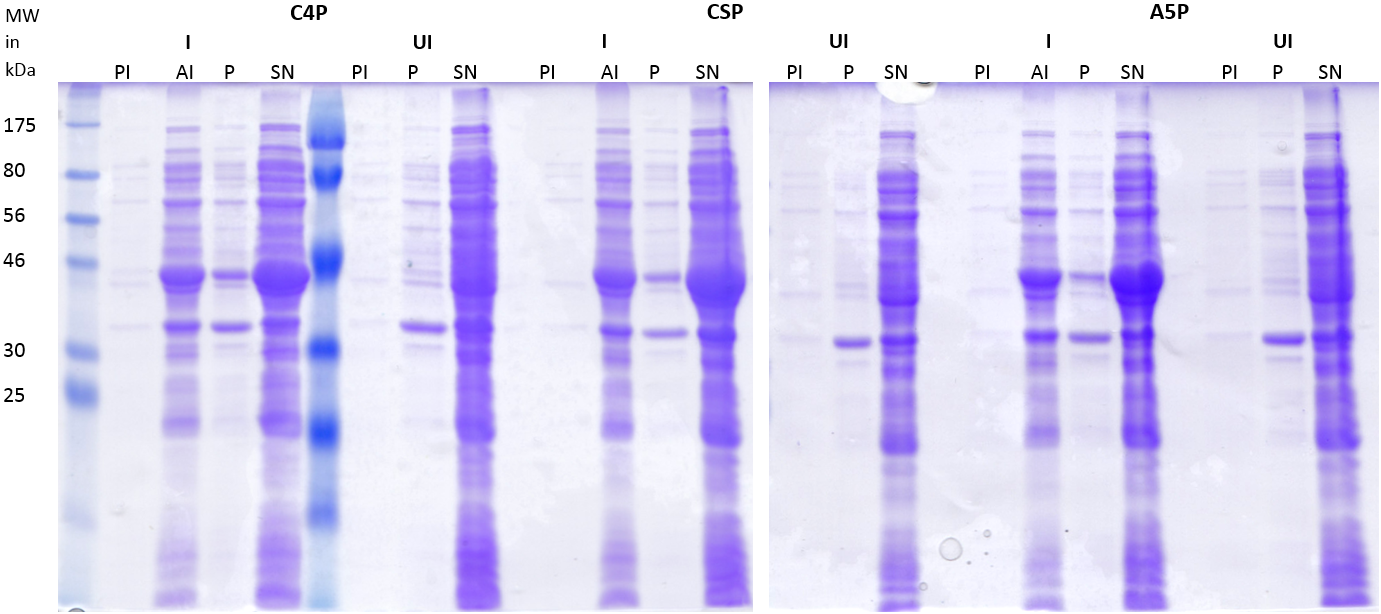
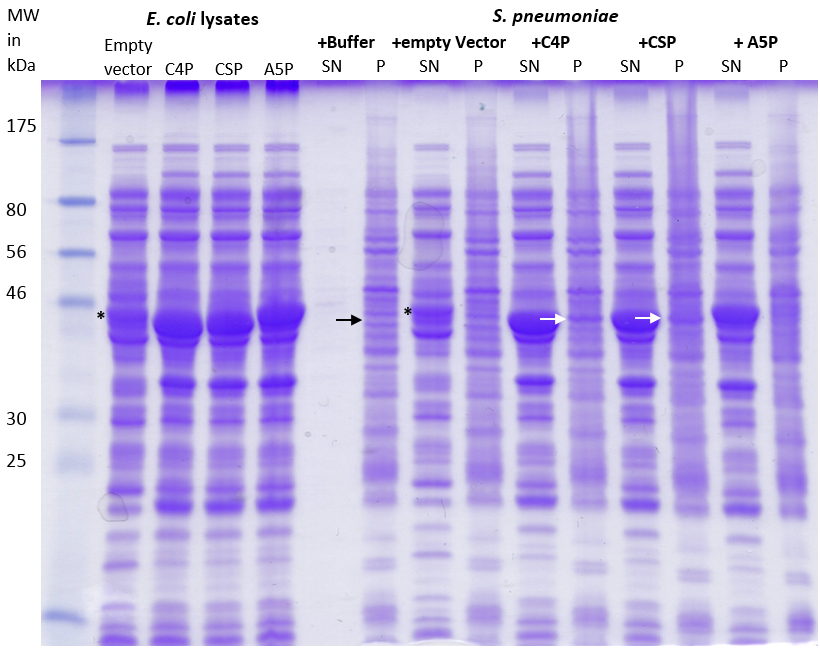
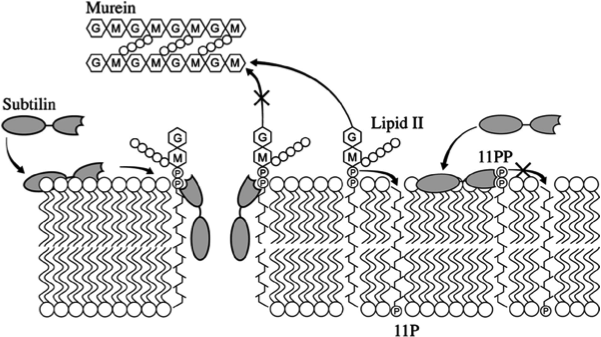
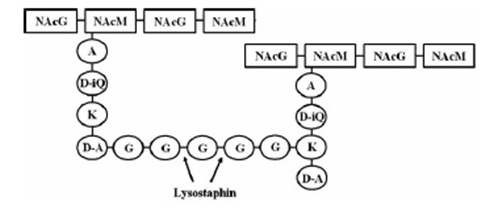
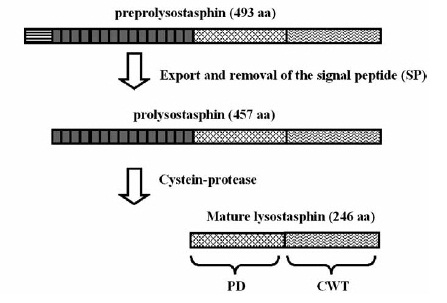
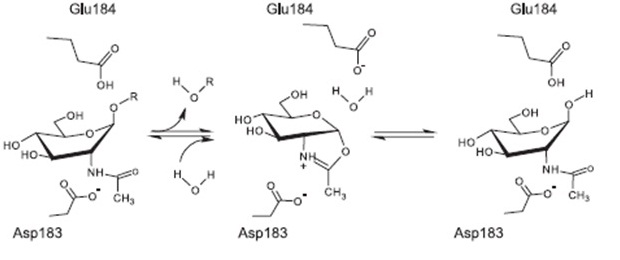
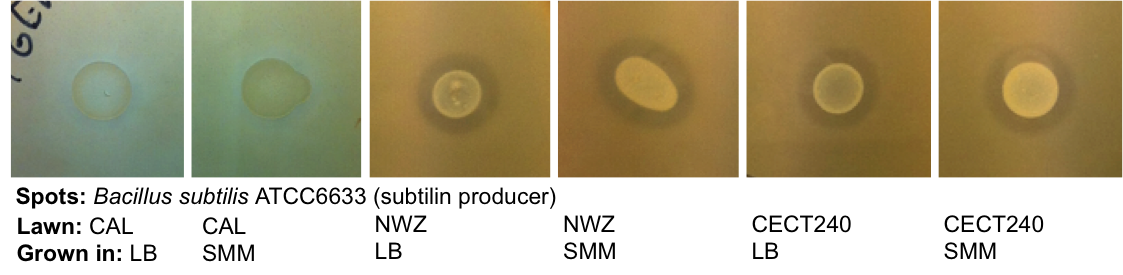
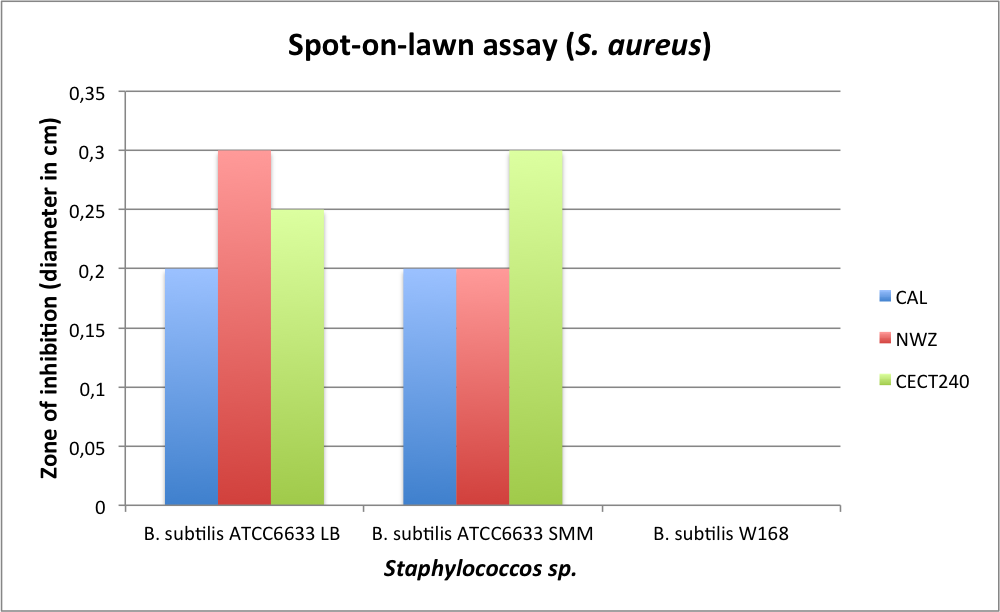
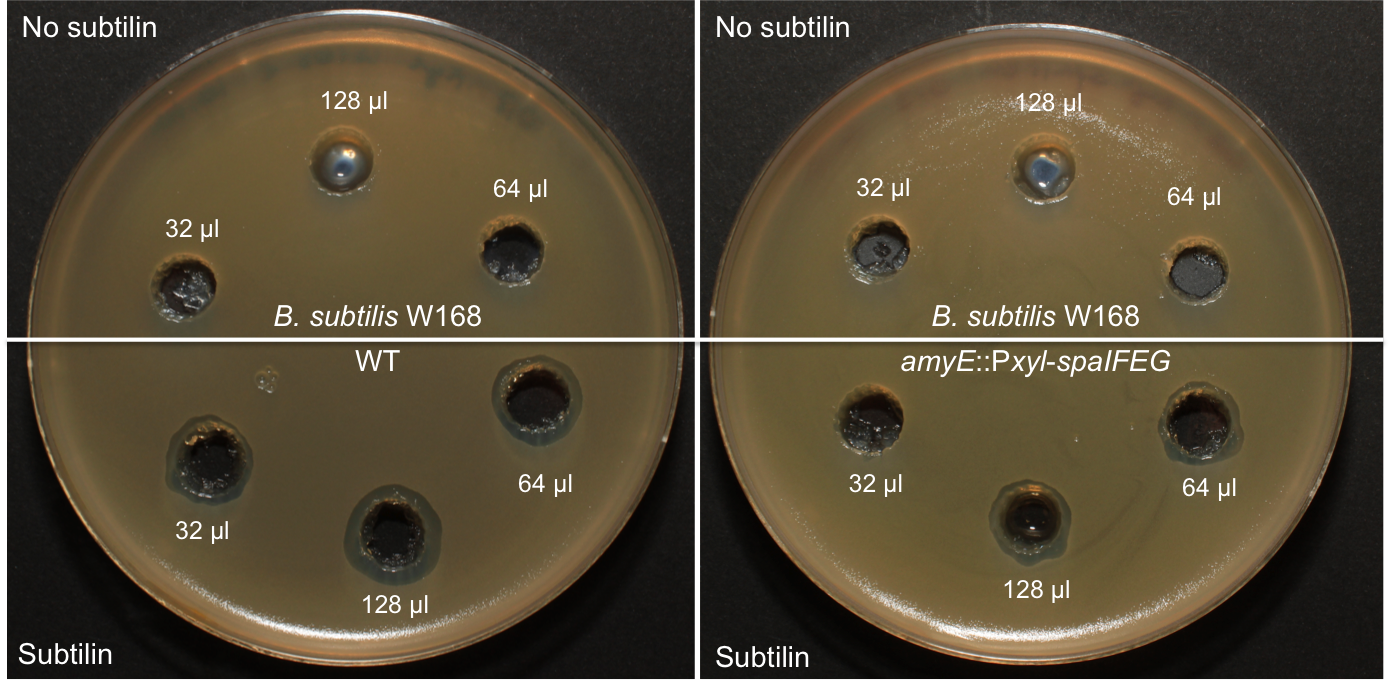
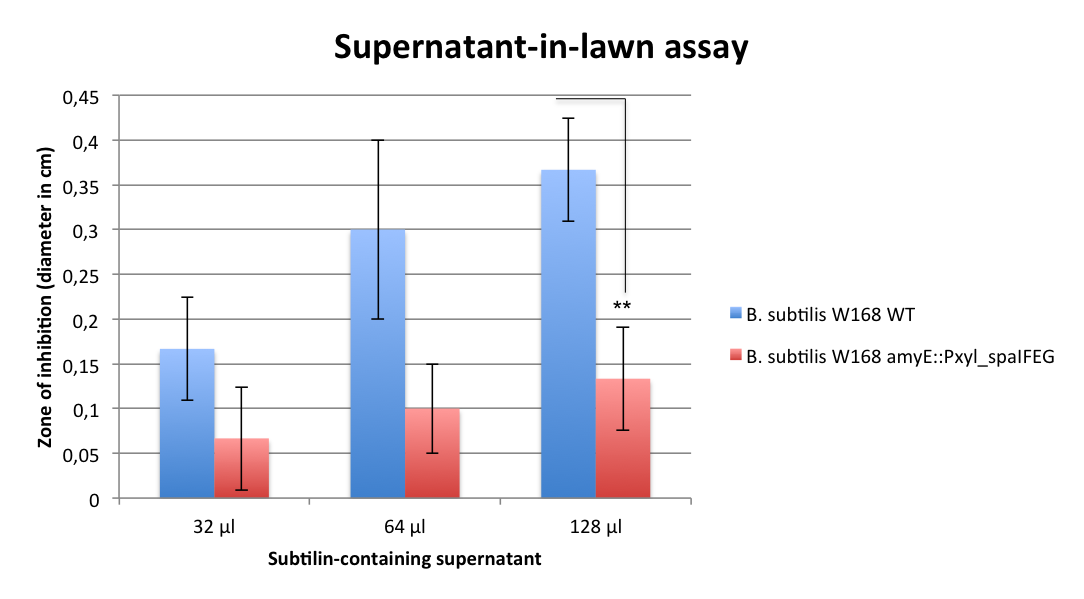
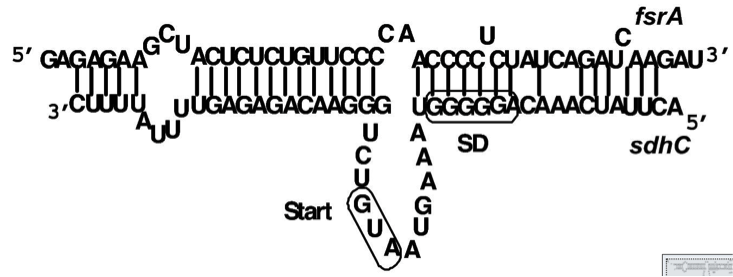
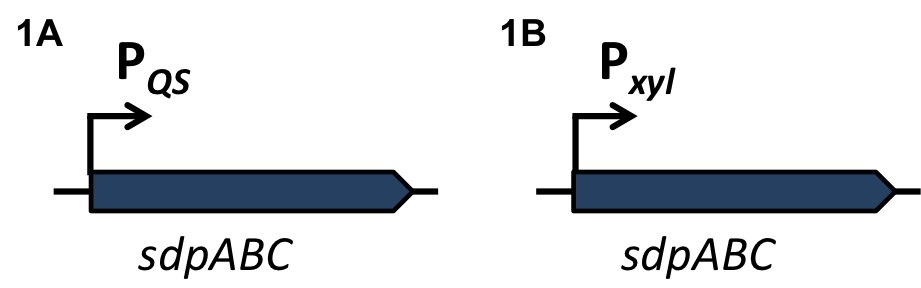
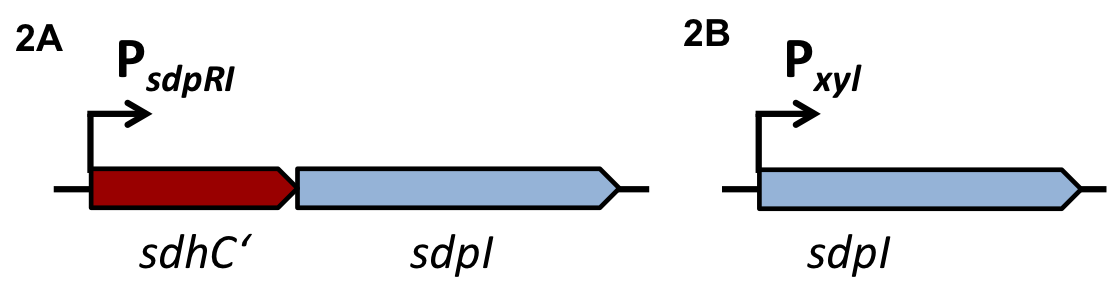
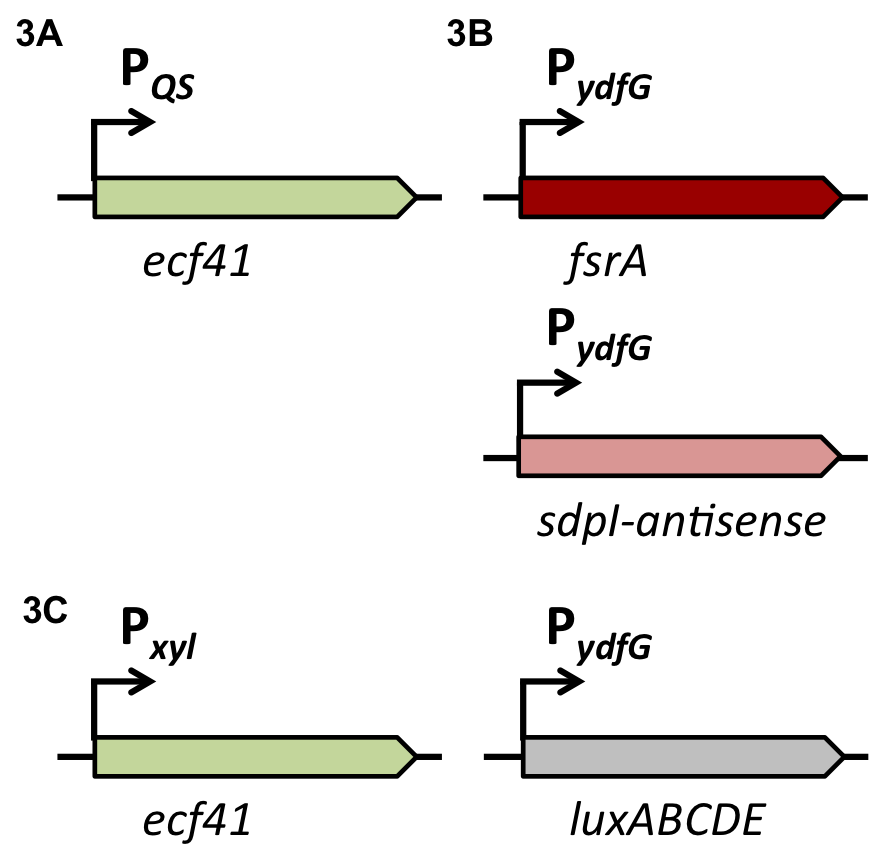
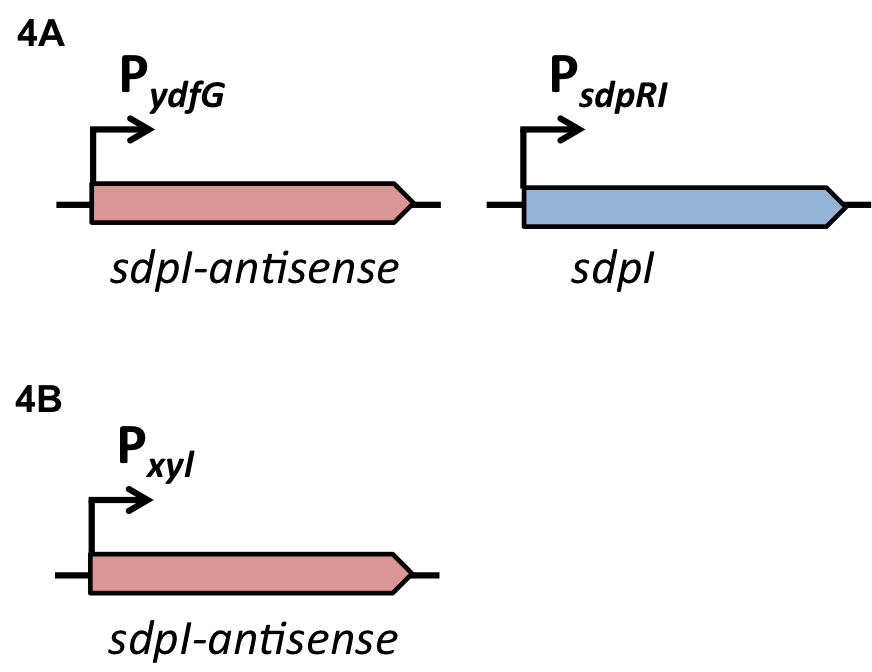
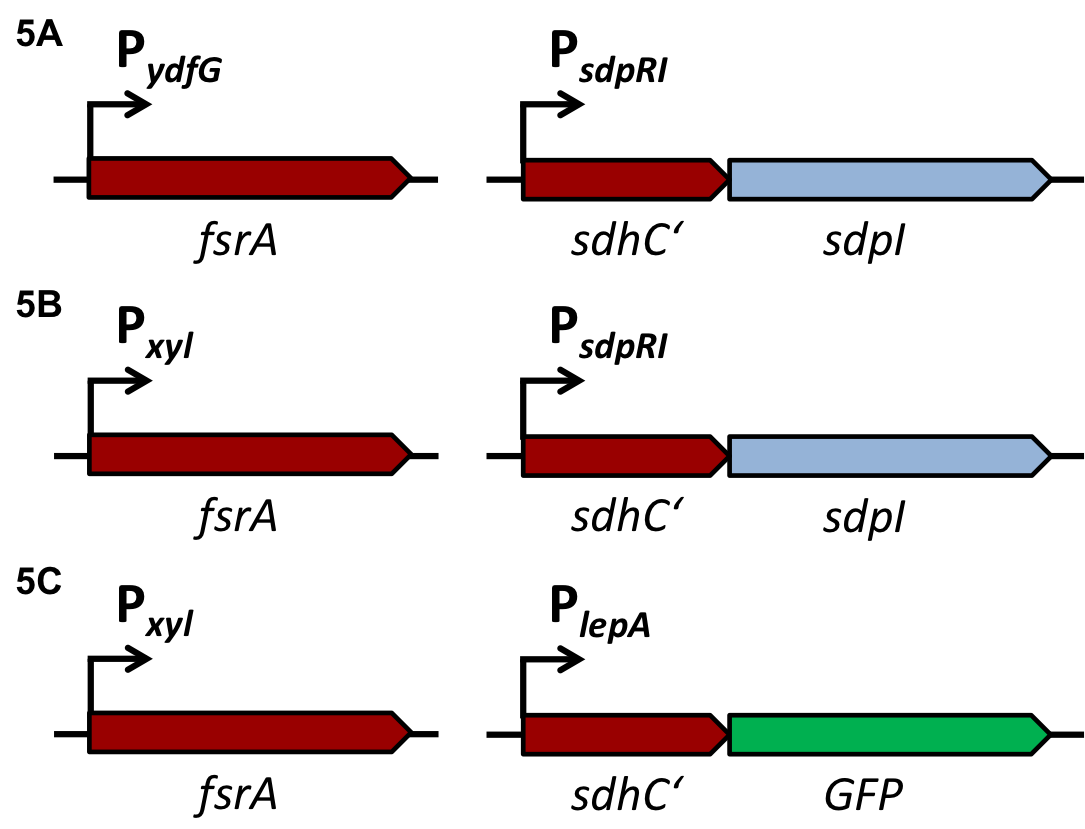
Staphylococcus aureus
Virulence of S. aureus
S. aureus is living as a commensal in humans with a prevalence of up to 30% of the population in Europe [http://www.nivel.nl/sites/default/files/ECCMID%202013%20-%20Resistance%20results_0.pdf (1)]. It is also responsible for a variety of diseases ranging from skin infections to life threatening toxinosis and is quickly adapting to the use of common antibiotics by becoming resistant. This dual lifestyle and the expression of a multitude of virulence factors is tightly regulated by a process termed quorum sensing [http://jb.asm.org/content/193/21/6020 (2)].
Quorum sensing in S. aureus
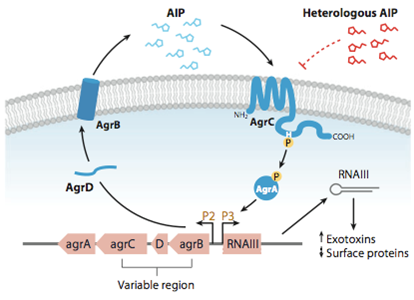
Quorum Sensing is a mechanism of bacterial cell-cell communication, granting bacteria the ability to measure the density of their surrounding cell population, and react to this by upregulation of specific genes. In S. aureus’’ this process is one of the major regulator of its virulence, making sure that energy-costly virulence factors are only produced when a large enough number of pathogens is present to overcome host-defence mechanisms.
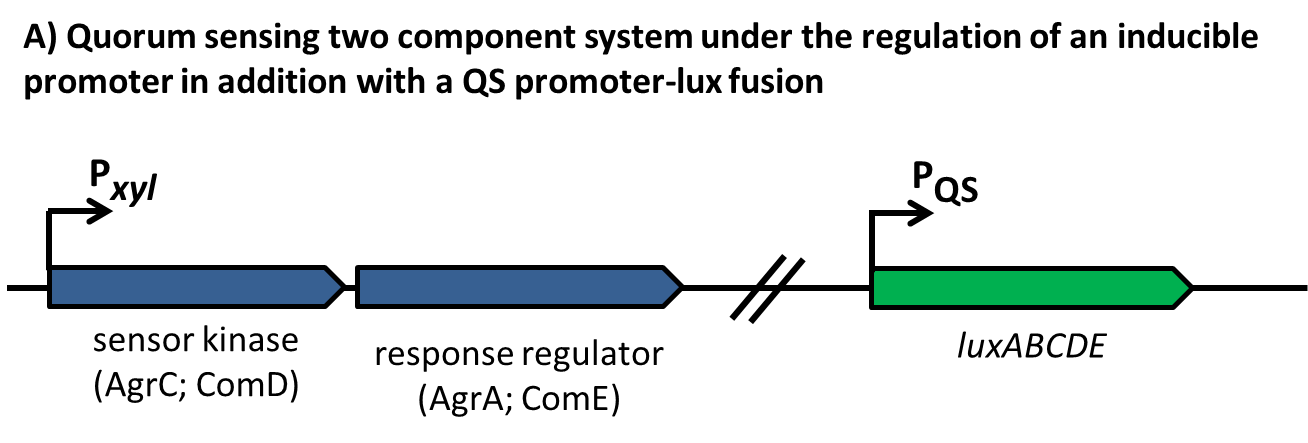
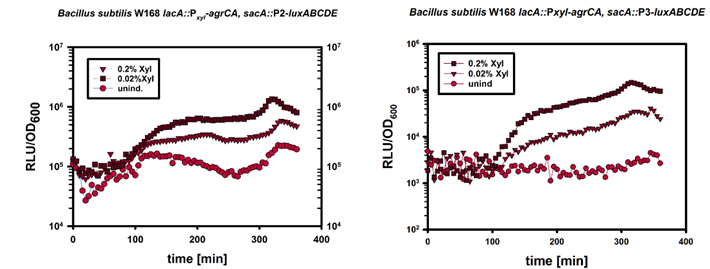
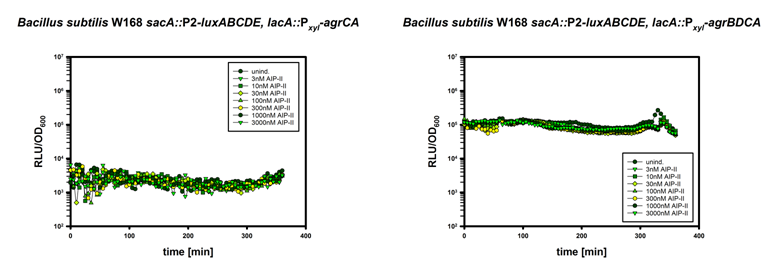
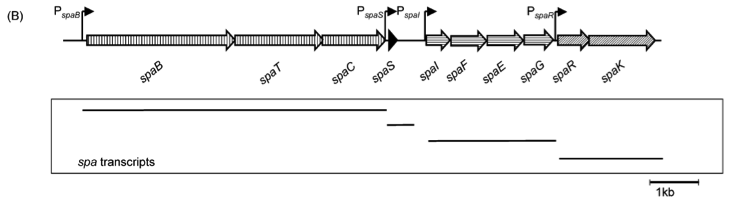
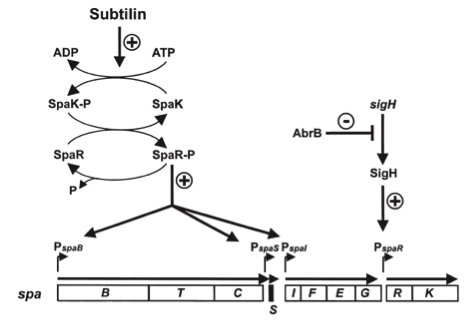
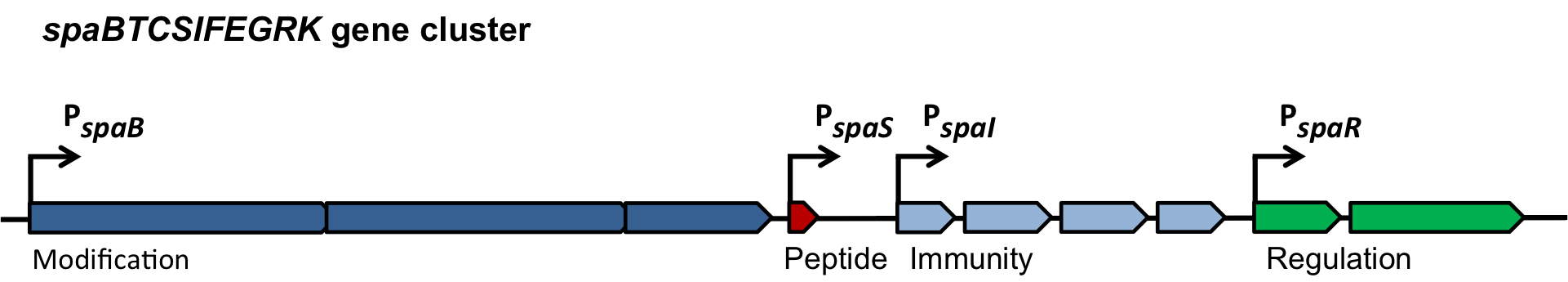
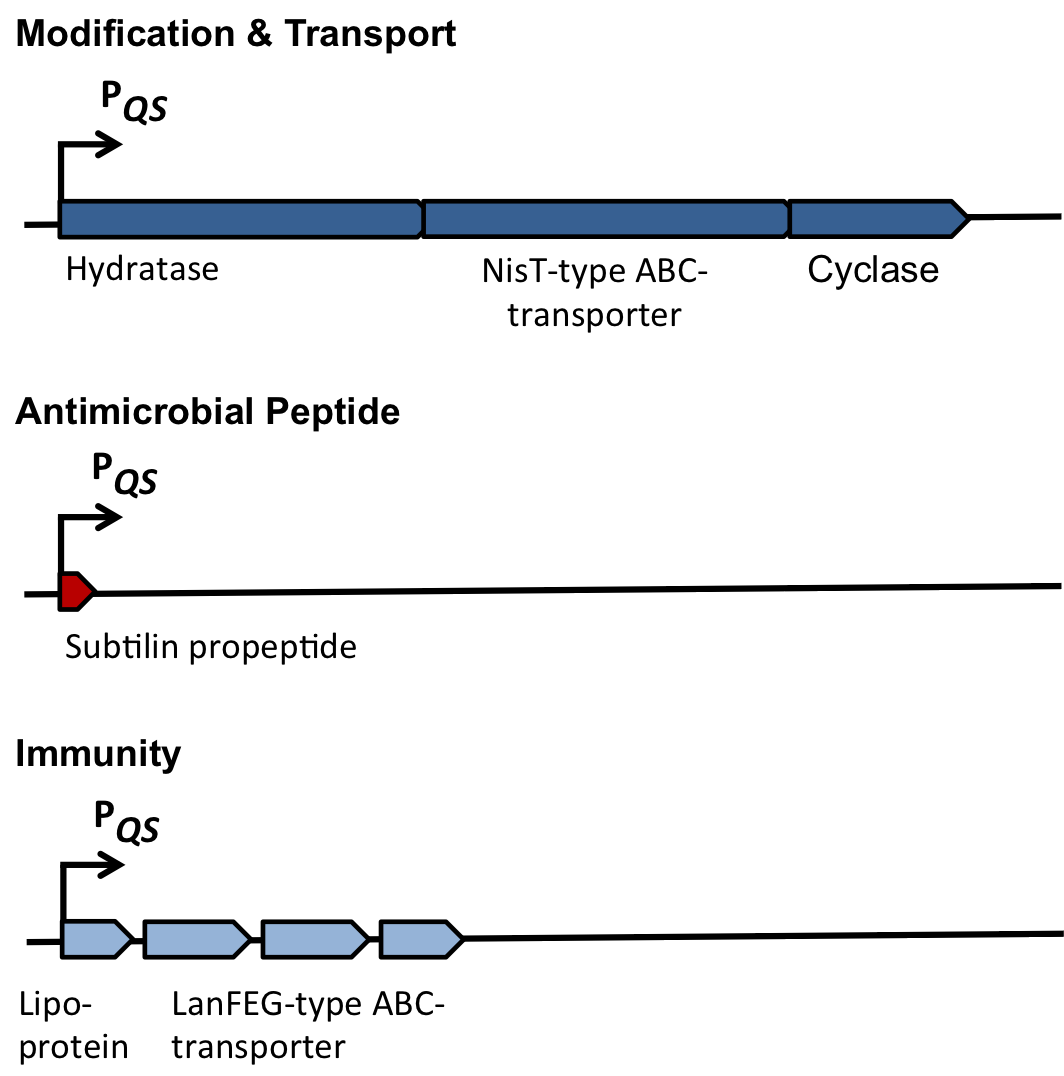
In this mechanism a certain peptide termed autoinducing peptide (AIP) is produced from a pro-peptide called AgrD and further processed and exported by a membrane protein AgrB to create a cyclic AIP. When a certain threshold concentration of AIP in the environment is reached, AIPs activate the receptor-histidine kinase AgrC. Upon induction AgrC phosphorylates the intracellular response regulator AgrA with its cytosolic histidine kinase domain [http://www.annualreviews.org/doi/abs/10.1146/annurev.genet.42.110807.091640 (3) ]. Phosphorylated AgrA then binds to distinct sites in the P2 and P3 promoter-region and activates these promoters. [http://jb.asm.org/content/193/21/6020 (2)] The P2 promoter activates transcription of the agrBDCA-Operon and thereby represents a positive feedback-loop. The P3 promoter activates transcription of the RNA-III transcript, involved of virulence gene expression [http://jb.asm.org/content/182/22/6517.full (4)]).
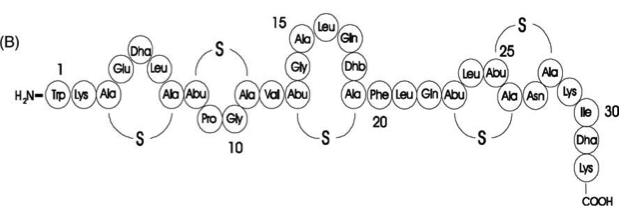
Interestingly, accumulated mutations in the hypervariable region of the agrBDCA-operon have led to four different pherotypes of S. aureus, using different AgrC-types, and producing different AIPs (AIPI, AIPII, AIPIII, AIPIV), which cross-inhibit each other and might correlate with certain diseases associated with S. aureus [http://www.sciencemag.org/content/287/5452/391.full.html (5)].
For this project we used genomic DNA derived from the strain Staphylococcus aureus N315, which is a MRSA strain, belonging to the AIP-II group.
Streptococcus pneumoniae
Virulence of S. pneumoniae
All over the world, Streptococcus pneumoniae causes the life threating invasive diseases pneumonia, sepsis and meningitis. In early childhood the diplococcus colonizes the epithelium of the upper respiratory tract as a commensal bacterium. Under certain circumstances, it gets a threat by tissue invasion. Still up to date, not much is known regarding the mechanism behind the shifting from a colonizer organism to an invader or how S. pneumoniae is able to cross the blood-brain barrier to cause meningitis.[http://www.ncbi.nlm.nih.gov/pubmed/19880020] To cross the line from an opportunist to a pathogen, S. pneumoniae needs to express specific virulence factors in a coordinated way. Apart from virulence factors like the polysaccharide capsule, the pore forming toxin pneumolysin or surface proteins like PspA and PspC, also biofilm production plays an important role for persistence in the human nasopharynx and contributes to pneumonia and meningitis [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1618759/].
One aspect of its infectiousness is the ability to perform natural transformation. Thus, it can take up extracellular DNA containing the information for resistance against diverse antibiotics and permanently incorporate the DNA into its genome via recombination. The main problem of treating S. pneumoniae infections is the increasing spread of ß-lactam resistance. Luckily, the molecular mechanism behind natural transformation in S. pneumoniae is the focus of current research.
Natural Transformation in S. pneumoniae
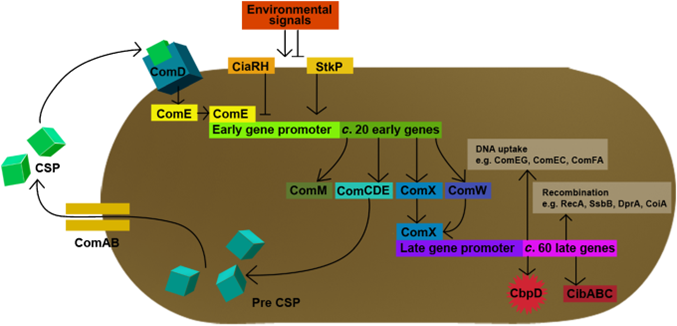
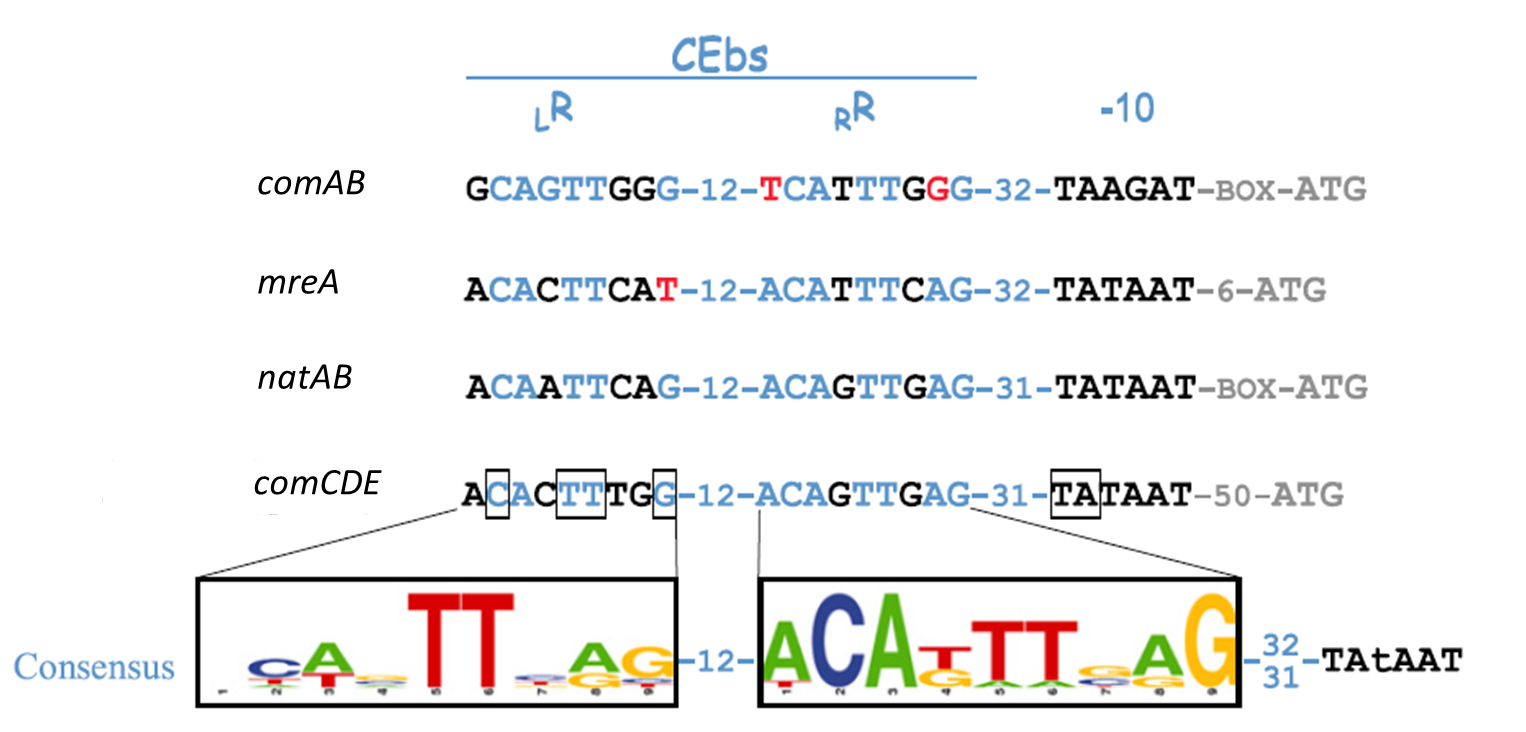
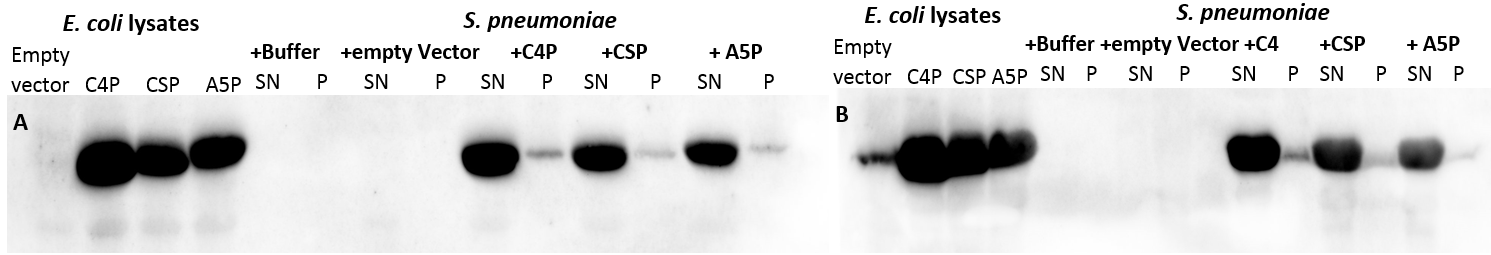
In S. pneumoniae, biofilm production and competence for natural genetic transformation are triggered by a two component regulatory system, responsive to environmental stimuli. There, a peptide pheromone called competence-stimulating-peptide (CSP) binds to and consequently activates the membrane-embedded sensor kinase ComD by changing the conformation of the polytopic kinase. ComD autophosphorylates and subsequently transphosphorylates the cognate response regulator ComE. ComE acts as transcription activator via binding to the -10 promoter regions of early and late competence genes. These ComE binding sites (CEbs) consist of a 9 bp direct repeat seperated by a stretch of 12 nucleotides.
A positive feedback loop ensures the availability of the two component system by activating the expression of the comCDE operon, where comDE encodes for the two component system ComDE and comC for the precursor peptide pre-CSP. Pre-CSP is cleaved to the mature peptide pheromone and transported to the extraplasmic room by the ABC transporter ComAB, whose expression is also activated by ComE. [http://www.ncbi.nlm.nih.gov/pubmed/?term=regulation+of+natural+genetic+transformation+and+acquisition+of+transforming+DNA]. In addition, ComE~P is able to trigger the expression of comX and comW , encoding for the alternative σ-factor X, and its stability factor ComW. This alternative σ-factor is required for the transcription of the so called late competence genes involved in the process of DNA uptake and recombination.
We are working with the genomic DNA of the nonencapsulated apathogenic strain R6 of S. pneumoniae, whose genome is completely sequenced [http://www.ncbi.nlm.nih.gov/pubmed/11544234]. CSP is a 17 amino acid long linear protein with the following amino acid sequence: Glu-Met-Arg-Leu-Ser-Lys-Phe-Arg-Asp-Phe-Ile-Leu-Gln-Arg-Lys-Lys-OH [http://www.microbesonline.org/cgi-bin/fetchLocus.cgi?locus=134925&disp=4]with a charged N-terminal residue and a positively charged C-terminal tail [http://www.ncbi.nlm.nih.gov/pubmed/16484185].
Sources
[1] den Heijer, C. D., van Bijnen, E. M., Paget, W. J., Pringle, M., Goossens, H., Bruggeman, C. A., . . . Team, A. S. (2013). Prevalence and resistance of commensal Staphylococcus aureus, including meticillin-resistant S aureus, in nine European countries: a cross-sectional study. Lancet Infect Dis, 13(5), 409-415. doi: 10.1016/S1473-3099(13)70036-7
[2] Reyes, D., Andrey, D. O., Monod, A., Kelley, W. L., Zhang, G., & Cheung, A. L. (2011). Coordinated regulation by AgrA, SarA, and SarR to control agr expression in Staphylococcus aureus. J Bacteriol, 193(21), 6020-6031. doi: 10.1128/JB.05436-11
[3] Novick, R. P., & Geisinger, E. (2008). Quorum sensing in staphylococci. Annu Rev Genet, 42, 541-564. doi: 10.1146/annurev.genet.42.110807.091640
[4] Jarraud, S., Lyon, G. J., Figueiredo, A. M., Lina, G., Vandenesch, F., Etienne, J., . . . Novick, R. P. (2000). Exfoliatin-producing strains define a fourth agr specificity group in Staphylococcus aureus. J Bacteriol, 182(22), 6517-6522.
[5]
[5] van der Poll, T., & Opal, S. M. (2009). Pathogenesis, treatment, and prevention of pneumococcal pneumonia. Lancet, 374(9700), 1543-1556. doi: 10.1016/S0140-6736(09)61114-4
[6] Oggioni, M. R., Trappetti, C., Kadioglu, A., Cassone, M., Iannelli, F., Ricci, S., . . . Pozzi, G. (2006). Switch from planktonic to sessile life: a major event in pneumococcal pathogenesis. Mol Microbiol, 61(5), 1196-1210. doi: 10.1111/j.1365-2958.2006.05310.x
[7] Johnsborg, O., & Havarstein, L. S. (2009). Regulation of natural genetic transformation and acquisition of transforming DNA in Streptococcus pneumoniae. FEMS Microbiol Rev, 33(3), 627-642.
[8] Hoskins, J., Alborn, W. E., Jr., Arnold, J., Blaszczak, L. C., Burgett, S., DeHoff, B. S., . . . Glass, J. I. (2001). Genome of the bacterium Streptococcus pneumoniae strain R6. J Bacteriol, 183(19), 5709-5717. doi: 10.1128/JB.183.19.5709-5717.2001
[9] Sequence obtained from http://www.microbesonline.org
[10] Johnsborg, O., Kristiansen, P. E., Blomqvist, T., & Havarstein, L. S. (2006). A hydrophobic patch in the competence-stimulating Peptide, a pneumococcal competence pheromone, is essential for specificity and biological activity. J Bacteriol, 188(5), 1744-1749. doi: 10.1128/JB.188.5.1744-1749.2006
 "
"